What’s the holdup on COVID vaccines for kids 4 and younger?
The FDA’s surprise announcement Friday set the expected schedule for vaccine eligibility for the youngest Americans back several months.
Listen 5:26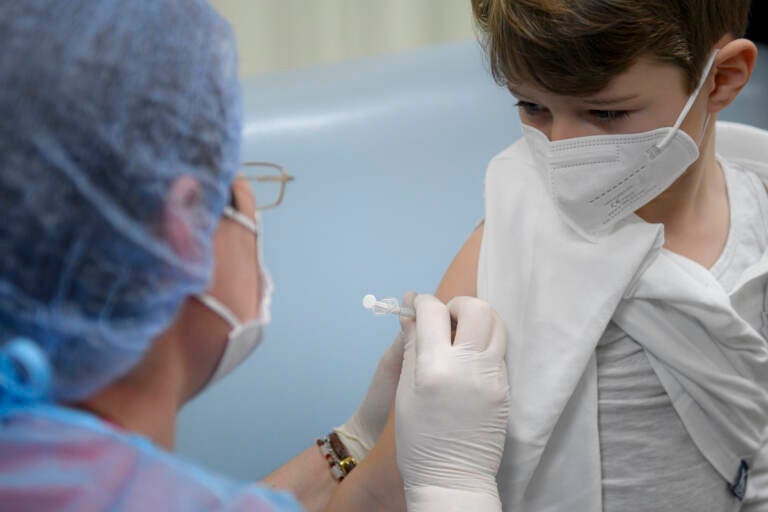
Alexis, 6 years old, gets his first dose of the Pfizer-BioNTech COVID-19 vaccine for children 5 to 11 years old, at a vaccination center in Bucharest, Romania, Wednesday, Jan.26, 2022. (AP Photo/Andreea Alexandru)
Ask us about COVID-19: What questions do you have about the coronavirus and vaccines?
This is one of a series of articles in which reporters from WHYY’s Health Desk Help Desk answer questions about vaccines and COVID-19 submitted by you, our audience.
Minh-Tu Do and his wife were relieved in January when they learned their 4-year-old son Joey received the COVID-19 vaccine and not a placebo during Pfizer’s pediatric clinical trial at Rutgers University last summer. (Their older son, 9-year-old Justin, who was also in the trial, received the actual vaccine too. They got that news in December.)
After hearing the good news about Joey, the family took a trip to the Nickelodeon Universe theme park in East Rutherford, New Jersey. They had held off going to any theme park during the pandemic, but knowing their 4-year-old was vaccinated helped ease their worries.
“It looked like they had the most fun they’ve had in the last two years,” said Do, a pediatric emergency medicine physician. “I could tell they were smiling through their masks by the way they were interacting with each other, running all around the place and things like that.”
But kids younger than 5 who were not part of the clinical trials will have to wait a bit longer for their shots. On Friday, the Food and Drug Administration reversed a previous decision and postponed the approval process for Pfizer’s COVID-19 vaccine for children 4 and under until April.
The FDA had planned to meet Tuesday to review data on a two-dose regimen for young children that is one-tenth of the adult doses. Ten million doses were expected to be shipped if the vaccine was approved. But now, those officials want to wait until data on a third dose is complete. The agency’s statement to the press did not make clear what had led to that decision.
Clinical trials showed that 2- to 4-year-olds did not achieve the desired immune response — meaning their antibodies did not meet the standard of other age groups. Pfizer launched a trial to study a third dose among children in this age group, hopeful that will achieve the desired immune response.
The approval process for two doses was originally planned for this week, to give kids under 5 a head start. Though health experts admitted that was unusual, the idea was that children could get at least some protection in the meantime, and that by the time a third dose would be approved around April, the kids would be ready for it, having had their second dose already.
“This age group of kids have been without any vaccine protection, and with the omicron surge, a lot of these kids were getting infected, and the number of kids being hospitalized and getting sick from COVID has been rising in the last couple of months,” Dr. Sunanda Gaur, a professor of pediatrics at Rutgers, said of the previous FDA plan to start the approval process sooner.
Dr. Jonathan Miller, medical director of value-based care and chief of primary care at Nemours Children’s Hospital, Delaware said that though he is concerned that 2- to 4-year-olds had lower antibody levels than other age groups, any immune response is better than none.
He said he’s also confident a third dose will achieve the optimal immune response. So, Miller argued, kids would benefit from getting their first doses now while the third dose trial is ongoing.
“I thought it made sense given the urgency around omicron to roll the vaccine out now for infants and toddlers. I’d love to understand why they would like to postpone it at this point,” he said.
“It means all those kids continue to be at significant risk of acquiring and spreading COVID,” Miller said. “It means that the day cares and child care centers that continue to close down because of COVID spread in their locations are going to continue to deal with those issues. And it probably means the pandemic goes on longer because we still have a significant part of our population that remains unvaccinated. So it is disappointing.”
Gaur, who leads the pediatric COVID-19 vaccine trials at Rutgers, said she was surprised by the news. But she said she also understands that the FDA wants to see the full data, and she recognizes that decisions are ever evolving in the midst of a pandemic.
“One way to look at it is we are in unusual times, and we are seeing unusual actions occurring, and we just have to learn to go with the flow a little bit,” Gaur said.
“Obviously, FDA and Pfizer made this decision that it’s probably better to get all the data in as it would have been in normal times. I am not privy to all the data at all as to what led to this change,” she said. “So all I can say is it’s interesting — it just makes for a lot of confusion for parents, and I feel for that. It’s a little bit of a roller coaster — you start to become excited that, ‘Oh, well, there’s an opportunity to at least get the vaccine series started,’ and now it’s like, ‘OK, we’ve got to wait some more.’”
Clinical trials also showed that children between 6 months and 2 years old did produce sufficient antibodies after vaccination.
Miller and Gaur said they don’t know why the FDA is not continuing the approval process for that age group. Gaur surmised that it could be part of an effort to schedule vaccines in a uniform manner, to avoid errors. Miller said he wishes the FDA had moved to vaccinate that group.
What do physicians and scientists know now?
Clinical data shows that antibodies produced by the vaccine were lower among 2- to 4-year-olds than any other age group. But researchers do not yet know why, or what that would mean in real-world scenarios.
Researching immune responses is not the same as evaluating vaccine efficacy. The clinical trials studied how the vaccine induces the production of antibodies, which act like a shield to block out viruses. Those findings do not determine the chances that a person will get COVID-19.
“You really don’t know exactly what that cutoff of antibody level is that correlates with protection from infection. We have a better idea of what correlates with protection from severe disease and death,” Gaur said. “But there is a cutoff that is used as a standard cutoff in all these studies, and the 2- to 4-year age group did not reach that.”
She added that the study did not measure T cells, which also are activated by the vaccine and can help fight infection. It’s possible that two doses of the vaccine did spark optimal T cells.
Enrollment for the third-dose clinical trial has begun, including at the site at Rutgers University. The interval between the first two doses is three weeks, and the interval between dose two and Dose Number Three is any time beyond 60 days, Gaur said.
Parent Minh-Tu Do, who is a pediatrician himself, said he was somewhat disappointed to learn son Joey needed a third dose, but not surprised — not everything goes perfectly in trials.
“If a study in the next few months shows that a third dose does, in fact, increase the antibody protection to a level comparable to the adults and the older children, and they do recommend it nationally, then definitely 100% I would back that up and recommend it to my patients or my family friends who have children of that age group,” he said.
Should I vaccinate my young children once they’re eligible?
The clinical trials for this age group have shown a strong safety level. There were no cases of adverse events such as myocarditis or blood clots.
Common side effects have tended to be lower as well, because of the smaller doses. Kids under 5 receive just one-tenth of the adult dose — 5- to 11-year-olds receive one-third of the adult dose.
“What is the risk of COVID disease for this age group? We have to balance that against the sort of minimal risk of the vaccine. And the risk of the disease is still pretty significant for these kids,” Miller said.
“With the omicron variant, we were seeing a lot of infants and toddlers get admitted to the hospital with symptomatic respiratory problems like croup related to COVID,” he said, referring to an upper-airway infection that causes a barking cough. “And a lot of these kids are spreading it to their older family members, who are definitely at higher risk from the virus. And so there’s still a portal of entry for families to get this disease, which can be devastating for some of the higher-risk and older people.”
Though there have been far fewer deaths among children than adults, they are not immune to COVID-19. On Jan. 16, almost 14% of emergency department patients with COVID in the United States were between 0 and 11 years old. At Nemours Children’s Hospital between November and January, 104 of the 185 pediatric patients hospitalized with COVID-19 were 5 and under.
“[In December], which was the omicron variant, I was seeing a decent percentage of my inpatients with COVID were infants and toddlers dealing with symptomatic respiratory symptoms that required being hospitalized,” Miller said. “And that was very different from what I had been seeing with prior variants, which just sort of highlighted how the changing nature of coronavirus seems to be impacting younger kids more and more over time.”
“And that worries me,” Miller said, “that whatever comes with the next wave — and I think we should assume that there’s going to be another wave at some point in the not-too-distant future —- I anticipate that’s going to impact kids significantly the way each of these waves has gotten worse for kids. And so vaccinating now for this age group is a really good way of trying to protect your kids from whatever the next wave is.”
Still, many kids who are eligible are not being vaccinated. Just over 31% of 5- to 11-year-olds in the U.S. have received at least one dose. A Kaiser Family Foundation COVID-19 Vaccine Monitor survey found that just three in 10 parents would get their children vaccinated as soon as it became available.
“I think that I would really like to encourage the parents to consider vaccinations, it’s very important. It’s really one way to protect the kids. Kids are still getting sick from this virus. It’s not as benign as people think. Kids do get very sick, and they do end up in the ICU,” Gaur said. “My plea would be that the vaccine is very safe and effective. So let’s please vaccinate kids. The kids that get in [the hospital] and are admitted, the vast majority are unvaccinated.”
How do I protect my little kids until April?
Gaur and Miller said they believe it’s important to continue to enforce masking for children over 3 until they can get vaccinated. States like New Jersey and Delaware have plans to lift mask mandates in schools, but Gaur and Miller said that decision should be halted, especially for unvaccinated preschool kids.
“I feel like the pediatric population has been overlooked time and time again throughout this pandemic when big public health decisions are made,” Miller said. “And this is just another example of that, where you have a whole group of people who are vulnerable right now under 5 years old. And yet states are deciding to lift mask mandates, which only puts that group at higher risk. So it’s sort of disappointing that we keep making these types of decisions, really only thinking about adults and the impact on adults.”
Though Do thinks that his son Joey is much safer at preschool now that he’s had two doses of the vaccine, he’s nervous about New Jersey lifting its mask mandate.
“I’m still debating with my wife whether or not having the vaccine, regardless of what his levels of antibodies are and whether or not he’s going to get the third dose anytime soon, I’m not sure if I’m still ready to send him to school without a mask, despite the schools having a new mandate to not require a mask any more,” Do said.
Miller and Gaur also advise parents to ensure that everyone in the household who is eligible for the vaccine gets their shots, and they recommend avoiding large gatherings.
“All adults that are in day care situations and families should really be vaccinated in order to protect the youngest ones from getting it,” Gaur said.
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.






![CoronavirusPandemic_1024x512[1]](https://whyy.org/wp-content/uploads/2020/03/CoronavirusPandemic_1024x5121-300x150.jpg)