Now, kids under 12 are getting their shot at COVID-19 vaccine clinical trials
A Pfizer clinical trial in younger kids is underway at Rutgers. WHYY’s Health Desk Help Desk talked to two families who signed up.
Listen 3:33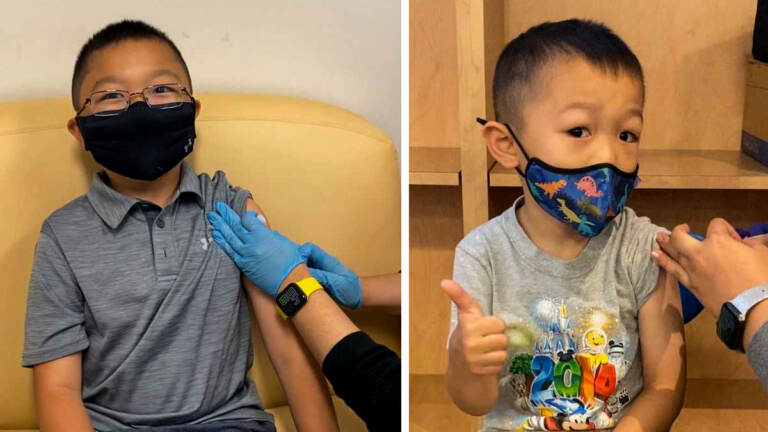
(Left to right): Brothers Justin Do, 9, and Joey Do, 4, of New Brunswick, have received two doses of the Pfizer vaccine, or a placebo, as part of a COVID-19 vaccine trial for kids under 12 at Rutgers University. (Courtesy of Minh-Tu Do)
Ask us about COVID-19: What questions do you have about the coronavirus and vaccines?
This is one of a series of articles in which reporters from WHYY’s Health Desk Help Desk answer questions about vaccines and COVID-19 submitted by you, our audience.
When Minh-Tu Do and his wife found an opportunity for their children Justin, 9, and Joey, 4, to get the COVID-19 vaccine as part of Pfizer’s pediatric clinical trial at Rutgers University, they jumped at the chance.
“I’m a proponent for them to get the influenza vaccine every year. So getting the COVID vaccine to me was not really a question of should we do it? It was more of a question of when can we get it?” said Do, who is a pediatrician.
Justin and Joey received two doses of the Pfizer shot each — or possibly two doses of a placebo — and will be followed for two years so researchers can track COVID-19 symptoms, side effects, and antibodies. It was important to contribute to science, Justin said.
“I was a bit nervous and a bit proud,” he remembered about getting the shot. “I was doing something that would make me safe and also that would help other people.”
Likewise, Nisha Gandhi said, her 10-year-old daughter Maya Huber wanted to participate in the trial. Gandhi applied to eight of them for her daughter, and landed in the one at Rutgers.
Gandhi is an ICU physician and her husband is an anesthesiologist. So they’ve been witness to a tragic year and a half.
“I saw lots of people with severe COVID and die from COVID, and so I feel strongly that the vaccine was helping to save lives and protect people from getting critically ill and dying from it,” she said.
Maya watched her parents leave for work to help sick patients and saw their worries about potentially catching the coronavirus themselves and spreading it to the family.
“Then when my husband and I were vaccinated back in December, we were so grateful and thankful, and believed that we would feel safe and that we would not do any harm to our kids,” Gandhi said. “And then Maya’s two older brothers, who are 14 and 13, they just got the vaccine in June, and they were excited that they would be vaccinated and be kind of free to live their best life now, with their friends and sleepovers, so Maya really wanted to be part of it.”
How the clinical trial works
Pfizer’s vaccine trials for children under 12 are taking place at 101 sites around the world. (Kids between 12 and 16 have been eligible to get the Pfizer shot since May.) The trial at Rutgers began mid-June and has enrolled about 65 kids so far.
Moderna, whose vaccine currently is only available for adults, began recruiting kids for its pediatric vaccine trial in March. In this region, Moderna is recruiting participants for a site at the Children’s Hospital of Philadelphia.
Kids must have parental permission and give their own assent, in which they agree to take part in the trial. Researchers explain the process, rights, and potential side effects to the kids and their families.
The children receive either two doses of the vaccine or two doses of a placebo, and they won’t know which one they got for six months. Those who get a placebo will have to get two additional shots if they want the real vaccine. For children between the ages of 5 and 12, the vaccine dose is lowered by one-third; it’s even lower for kids between the ages of 6 months and 2 years.
Over the next two years, the children will be carefully monitored for symptoms that could be related to the vaccine and are checked for COVID-type illness. Researchers also evaluate the children’s antibody responses, which involves drawing blood. A subset of children is participating in a study assessing how T and B cells function in response to the vaccine.
Data shows that children get COVID-19 less frequently than adults and are less likely to get severe illness from COVID-19 than adults. However, a significant number of kids have become sick with the coronavirus. More than 3.5 million children in the United States have tested positive for COVID-19 and 526 of them have died.
There’s also a rare chance of multisystem inflammatory syndrome, or MIS-C, which typically occurs after a child has recovered from a COVID-19 infection, when the body’s immune response is ramped up. Many MIS-C patients don’t know they previously had COVID-19 because they were asymptomatic. The patient might not get symptoms for MIS-C until up to eight weeks after having the virus. MIS-C can cause serious gastrointestinal, heart, and neurological problems.
As of June, children under 18 accounted for 25% of all new COVID cases in the United States.
“So even though it’s not as prominent an issue with children as it is with adults, they’re certainly at risk of getting sick from it. So therefore, they also deserve to have a vaccine to protect themselves,” said Sunanda Gaur, professor of pediatrics at Rutgers Robert Wood Johnson Medical School.
And even if kids get mild or asymptomatic infections, they can spread the virus to other, more vulnerable people.
“There is a risk that they could bring bad outcomes to their family members and the community they are interacting with,” Gaur said. “So as such, they need to be protected from getting infection and spreading it to others as well.”
She said children are also an important part of achieving herd immunity — the concept being that as more individuals have protective immunity, you will encounter fewer people who can be infected, so the virus doesn’t continue to spread as rapidly because it’s not finding susceptible hosts.
“The higher the numbers of people that are vaccinated, the less the chances that the virus will continue to spread and greater the chances that we’ll be able to stop the virus in its tracks,” Gaur said, “because the virus is always looking for susceptible people, and as long as there are lots of susceptible hosts for the virus, it will continue to find sanctuary and continue to propagate.
“So in that sense, just to build up that herd immunity level, it’s also important that children get immunized to this.”
About 18% of 239 parents of preteens and teens polled last month said they want to wait some time before getting their kids vaccinated, according to a survey published by the Kaiser Family Foundation.
Yet interest in Rutgers’ pediatric trial was high, Gaur said.
“We weren’t sure whether people would be interested. But we were very surprised,” she said. “We sent out the flyers and the information through various channels to the community. And we have had so much interest in the vaccine study for children that we’ve had to turn people away … It’s been an overwhelming response in a sense.”
Young people and vaccines so far
Only seven, or 0.6%, of 1,131 young people ages 12 to 15 who got the vaccine in clinical trials reported severe adverse effects, but researchers determined none was related to the shot, according to a study published in the New England Journal of Medicine.
Among the 9.2 million children under 18 who have received at least one dose of the vaccine, there have been no deaths, according to the American Academy of Pediatrics.
In June, the Centers for Disease Control and Prevention reported that there were more than 300 verified cases of myocarditis, or inflammation of the heart muscle, among 12- to 29-year-olds in the U.S. believed to be related to the mRNA vaccines. That’s out of more than 140 million people in the country who had been fully vaccinated with the Pfizer and Moderna vaccines.
The condition was more common among young males. Health experts say the adverse event is very rare, and most patients recover. Many reported self-resolving symptoms. About 30% of parents in the Kaiser study said they will not vaccinate their children because of the rare side effect.
The participants in the trial at Rutgers have been made aware of the rare heart inflammation, and so far, none of the families have backed out, Gaur said.
“We’re very sensitive to these cases of myocarditis that have occurred. So in view of that, we are following the children very closely,” she said. “The participants report every single symptom in an electronic diary that we monitor. So we are in touch with them constantly and we know if any signal is there and then evaluate it.”
Gaur said she wants parents who are hesitant about their children to understand that the vaccine is safe.
“We are in the middle of a pandemic that we’ve not seen for a hundred years, and I think that as a community, the best way we know of getting out of this is through vaccinations. That is our best armamentarium against this,” she said. “Everyone should take responsibility for helping everybody else out, including themselves, to get vaccinated so that we can all come out of this, and that includes children, they’re part of the community.
“These vaccines have been studied extensively. They’ve not been shortchanged, just like any other vaccine that’s been studied in the past. And they are safe,” Gaur added. “I know that there’s all of these side effects, they keep cropping up, but those types of things have occurred with every vaccine in the past. It’s just that this one’s getting a lot of attention. That does not mean the vaccine is not safe. The vaccine is very safe and it’s been well studied.”
What happens now
Pfizer has said it expects to seek Food and Drug Administration authorization to give its vaccine to kids under 12 in September.
Gaur doesn’t believe emergency use authorization for the vaccine in kids will be available by the time school starts in September, and in the meantime, she recommends that children continue to wear masks. Local school districts are making decisions now about the return to in-person classes in the fall, with disagreement within some school districts about how to proceed on the issue of masks.
Rutgers clinical trial participant Justin Do said that after getting the shot, vaccine, or placebo, he felt pain in his arm for about three or four days, had body soreness, headache, and nausea. Joey had no side effects.
“We do feel a little weight has been lifted for one of them, Justin, because I have a strong suspicion that he did receive the vaccine, given that he experienced similar side effects that I had when I received the Pfizer vaccine,” Justin’s father, Minh-Tu Do, said.
“The younger one, we’re still in the unknown. And that’s why we’re also still a little bit worried about having him out and about. And we try as much as we can to protect ourselves when we go out, we try to avoid gatherings. And if we do go out, we wear a mask and try to distance ourselves,” Do said.
If the kids did get the real vaccine, it would mean more freedom for the entire family, Do said, as long as studies show the vaccine is protective enough against variants.
“If I do know that the whole family is now fully vaccinated and carrying strong enough antibodies to fight the coronavirus, I would be more reassured to travel, for example, to visit my family that’s out of state. My family themselves would be more acceptable for us to come visit them as well, so that we’re not carrying a potential threat to them,” he said.
“As of right now, I am having my child playing soccer, but I’m making him wear a mask on the field even though it’s no longer required. If I know he’s vaccinated, I might be more inclined to allow him to not wear his mask while on the field.”
Justin has big plans too.
“I’m excited that I will be able to go to places that will probably be dangerous with COVID. But if I’m protected with a mask and the vaccine, I will be able to do fun things, like go to water parks or Disney World,” he said.
Similarly, Nisha Gandhi doesn’t know whether daughter Maya received the vaccine or the placebo. She’s making sure Maya wears her mask.
“I think everyone needs to do what’s important for them, themselves, and their family. I strongly feel that if this study shows that the side effects for children are minimal to nothing, that the risks of the vaccine are nothing compared to the risks of getting the virus itself … I’m waiting to see, but I’m cautiously optimistic that this will be a safe vaccine, and if that pans out, then I would highly recommend it for everyone to get vaccinated,” Gandhi said.

Get daily updates from WHYY News!
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.






![CoronavirusPandemic_1024x512[1]](https://whyy.org/wp-content/uploads/2020/03/CoronavirusPandemic_1024x5121-300x150.jpg)