Spark’s gene therapy for rare form of blindness wins FDA approval
For Katherine High, president and chief scientific officer at Spark Therapeutics, the drug’s approval signals renewed hope in the possibilities of gene therapy.
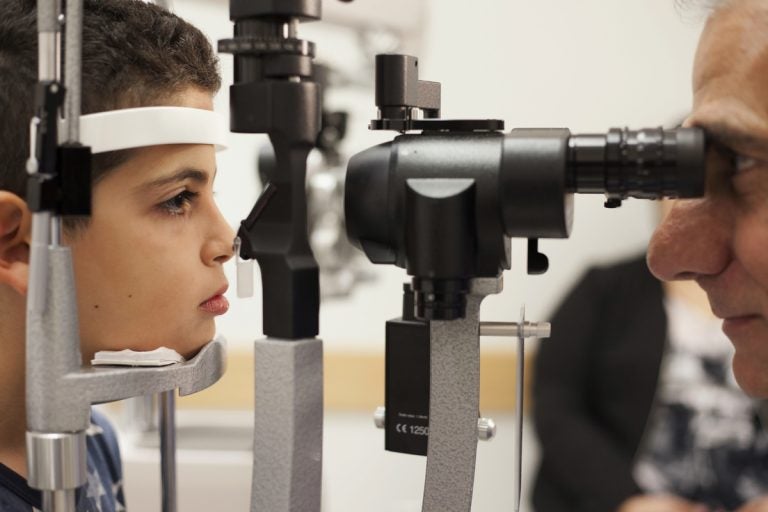
In this Oct. 4, 2017, file photo, Dr. Albert Maguire, right, checks the eyes of Misa Kaabali, 8, at the Children's Hospital of Philadelphia. Misa was 4-years-old when he received his gene therapy treatment. On Tuesday, Dec. 19, 2017, the Food and Drug Administration approved the therapy which improves the vision of patients with a rare form of inherited blindness, another major advance for the burgeoning field of genetic medicine. (Bill West/AP Photo, file)
Marking a major advance in the emerging field of genetic medicine, the U.S. Food and Drug Administration Tuesday approved the country’s first gene therapy for treating an inherited disease.
The Luxturna therapy improves vision for patients with a rare form of blindness called Leber congenital amaurosis that affects between 1,000 and 2,000 people in the U.S.
Spark Therapeutics in Philadelphia developed the therapy in collaboration with researchers at the University of Pennsylvania and Children’s Hospital of Philadelphia.
Luxturna works by delivering the correct copy of a missing gene through injection into a patient’s eyes. Once the gene takes up residence in the patient’s cells, it begins producing a protein that had been missing and corrects a biochemical pathway — allowing the patient to see better.
“Today’s approval marks another first in the field of gene therapy — both in how the therapy works and in expanding the use of gene therapy beyond the treatment of cancer to the treatment of vision loss — and this milestone reinforces the potential of this breakthrough approach in treating a wide-range of challenging diseases,” FDA Commissioner Scott Gottlieb said in a statement.
One patient shared a success story during the FDA approval process that has stayed with Katherine High, president and chief scientific officer at Spark Therapeutics.
“She talked about the first time that she saw rain falling from the sky,” High recalled. “And that she knew that if you walked in the rain you got wet, but she would never actually see the rain falling from the skies.”
For High, the drug’s approval signals renewed hope in the possibilities of gene therapy.
“The long journey to turn genes into medicine is becoming reality,” she said. “And that will spell more treatment options for people who were born with serious genetic diseases.”
Spark has not yet said how much the gene therapy will cost, but a similar drug in Europe was priced at around $1.2 million. Spark plans to release pricing information in January.
Disclosure: Spark Therapeutics CEO and co-founder Jeffrey Marrazzo is the son of WHYY CEO Bill Marrazzo.
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.