Abortion pills that patients got via telehealth and the mail are safe, study finds
Access to the abortion drug mifepristone could soon be limited by the Supreme Court for the whole country. Here, a nurse practitioner works at an Illinois clinic that offers telehealth abortion. (Jeff Roberson/AP)
In March, the Supreme Court will hear a case about mifepristone, one of two drugs used in medication abortions. A key question in that case is: Was the Food and Drug Administration correct when it deemed the drug safe to prescribe to patients in a virtual appointment?
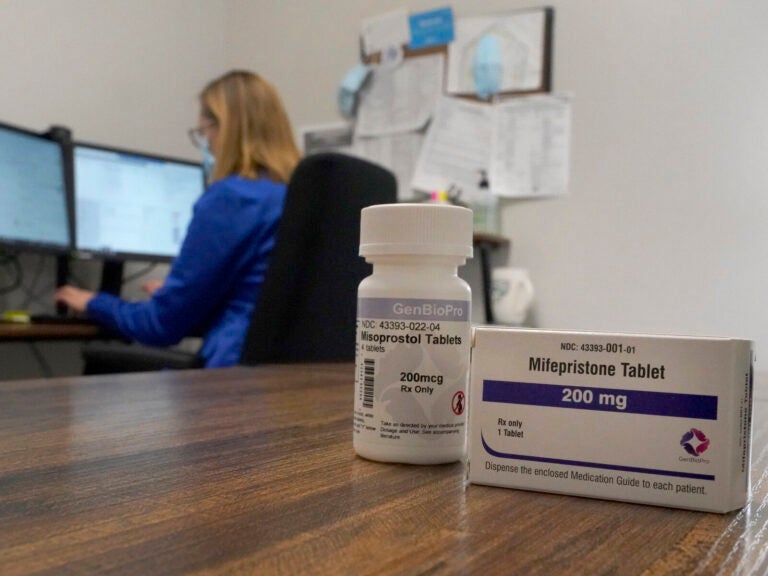
A study published Thursday in Nature Medicine looks at abortion pills prescribed via telehealth and provides more support for the FDA’s assessment that medication abortion is safe and effective.
Researchers examined the electronic medical records for more than 6,000 patients from three providers of abortion via telehealth. They also conducted an opt-in survey of 1,600 patients.
Some abortion patients talked to a provider over video, others used a secure chat platform, similar to texting. If patients were less than 10 weeks pregnant and otherwise found to be eligible, the providers prescribed two medications: mifepristone, which blocks a pregnancy hormone called progesterone, and misoprostol, which causes uterine contractions. Patients got both medicines via mail-order pharmacy.
“Then 3 to 7 days later, there was a clinical follow up,” explains the study’s lead author, Ushma Upadhyay of the University of California – San Francisco. “The provider checked in with the patient. ‘Did you receive the medications? Did you take the medications?’ They asked about symptoms. And then there was a clinical follow-up four weeks after the original intake.”
The researchers found that the medication was effective – it ended the pregnancy without any additional follow-up care for 97.7% of patients. It was also found to be safe – 99.7% of abortions were not followed by any serious adverse events. The safety and efficacy was similar whether the patients talked to a provider over video or through secure chat.
“These results shouldn’t be surprising,” Upadhyay says. “It’s consistent with the over 100 studies on mifepristone that have affirmed the safety and effectiveness of this medication.”
The results also echo international research on telehealth abortion and studies of medication abortion dispensed in a clinic with an in-person appointment, she notes.
Rishi Desai of Harvard Medical School is a medication safety expert who was not involved in the study. He says it was “well-conducted,” especially considering it can be difficult to track patients who only connect with providers remotely.
“I would say that this study provides reassuring data regarding safety of the medications, and this is very much in line with what we have seen in many previous studies,” he says. “So it’s good to see that safety findings hold up in this setting as well.”
Still, whether mifepristone is safe and whether FDA has appropriately regulated how it is prescribed is a live legal question right now.
An anti-abortion rights group sued FDA in 2022, arguing that mifepristone is not safe and was improperly approved in 2000. Judge Matthew Kacsmaryk, a district court judge appointed to the federal bench by President Trump, ruled that mifepristone should be pulled from the market nationwide. Although his decision didn’t take effect pending appeals, the appeals court ruled against the FDA in part, specifically rolling back telehealth abortion access. That is also on hold for now.
The Supreme Court hears arguments in the case on March 26. The decision could affect access to medication abortion nationwide and set a new precedent on challenges to the FDA’s authority.
Recently, there’s been a flurry of mifepristone research news. Last week, a paper that raised safety concerns about mifepristone was retracted. This study, released Thursday, affirms the FDA’s position that the medicine can be safely prescribed remotely.
Upadhyay says she’s been working on this research for years and that the timing of its publication weeks before the Supreme Court arguments is coincidental.
“I don’t know if they can enter new evidence into the case at this point,” she says. “But I do hope it impacts the perception of how safe this medication is.”




