Spark Therapeutics sets price for pioneering gene therapy
The Philadelphia-based company announced Wednesday that its recently-approved gene therapy, which has the potential to cure a rare form of blindness, will cost $850,000.
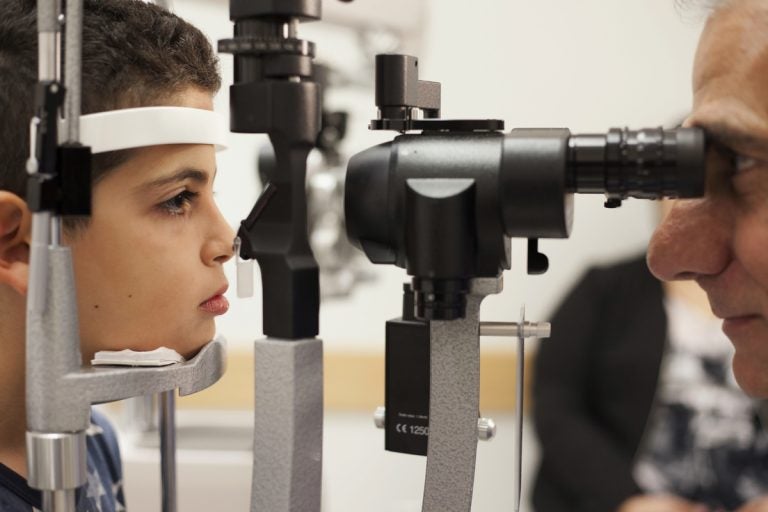
In this Oct. 4, 2017, file photo, Dr. Albert Maguire, right, checks the eyes of Misa Kaabali, 8, at the Children's Hospital of Philadelphia. Misa was 4-years-old when he received his gene therapy treatment. On Tuesday, Dec. 19, 2017, the Food and Drug Administration approved the therapy which improves the vision of patients with a rare form of inherited blindness, another major advance for the burgeoning field of genetic medicine. (Bill West/AP Photo, file)
Philadelphia-based Spark Therapeutics announced Wednesday that its recently-approved gene therapy, which has the potential to cure a rare form of blindness, will cost $850,000.
The gene therapy drug, Luxturna, was approved by the Food and Drug Administration in December and is the first gene therapy approved to treat an inherited genetic disorder. It’s been shown to restore vision in patients, often children, suffering from one form of a disorder called retinal dystrophy.
The $850,000 price tag for the one-time treatment actually comes in under the million dollars or more many analysts expected Luxturna to cost.
Spark CEO Jeff Marrazzo said the company’s own analysis showed that the value of restoring vision in these patients was on par with those estimates. But he said the lower price the company arrived at strikes a balance, in order to “address the access concerns that patients have, address the affordability concerns that payers have and the budgetary concerns that payers have, as well as our goal which is to develop a business model that can sustain our ability to continue to invest in this revolutionary science.”
Experts said the steep price is reasonable for this kind of curative therapy with benefits throughout a patient’s lifetime. Jeromie Ballreich, a health economist at the Johns Hopkins University Bloomberg School of Public Health, said that because the particular condition it treats affects only between 1,000 and 2,000 Americans, insurance companies shouldn’t struggle too much to be able to pay for the therapy.
“No one’s going to say this is a cheap drug by any means, but because it only affects such a small group of people, and provides such tremendous therapeutic value to these people, the costs can be spread across a larger population,” Ballreich said.
Ballreich said the real test for the affordability of expensive gene therapies may come when more drugs are invented that treat more common conditions. Then the costs really will start to add up, and insurers could be more hesitant to cover the therapies.
—
Disclosure: Spark Therapeutics CEO and co-founder Jeffrey Marrazzo is the son of WHYY CEO Bill Marrazzo.
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.