Possible treatment for COVID-19 enters clinical trial at Penn
Three separate sub-studies will launch next with the drug, widely used by people with autoimmune conditions like lupus and rheumatoid arthritis.
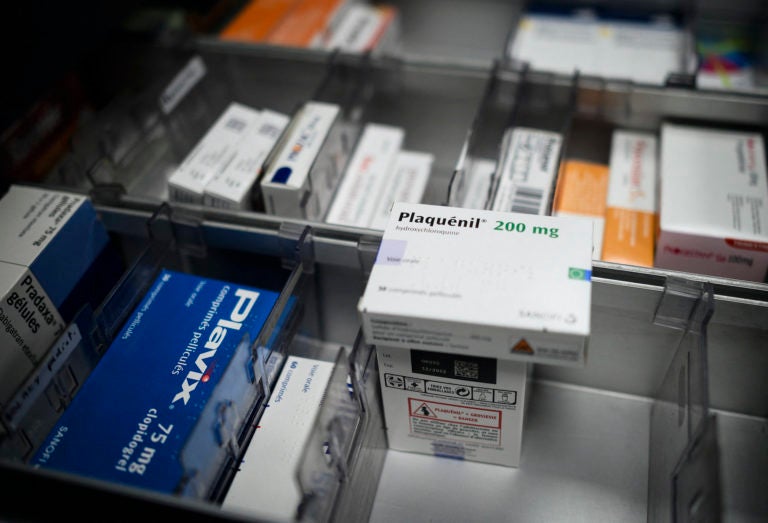
A box of Plaquenil, the brand name of hydroxychloroquine, is displayed on March 30, 2020 in Paris, France. (Eliot Blondet/Abaca/Sipa USA(Sipa via AP Images)
A new trial led by researchers at the University of Pennsylvania will test whether the drug hydroxychloroquine (HCQ) can help treat or prevent COVID-19.
The study, called “Prevention and Treatment of COVID-19 with HCQ (PATCH),” is currently enrolling patients in three separate sub-studies and is scheduled to launch next week.
Hydroxychloroquine is an antimalarial drug used regularly by people with autoimmune diseases. In lab tests, it has shown promise for preventing viruses from spreading on a cellular level, including in the viruses that caused the SARS and MERS epidemics, the properties of which are similar to the current coronavirus. Some research was published on the drug when those viruses were circulating, but after the epidemics died down, its popularity in the academic community waned.
When people across the globe began falling ill with COVID-19, it led to renewed interest in hydroxychloroquine among scientists — and to President Donald Trump hailing the medication as a game-changer before clinical trials had proved anything about its efficacy. As a result, people who desperately depend on the drug have had a hard time finding it, and incorrect use of it even led one Arizona man to his death. Now, researchers are testing the drug in rigorous clinical trials to see if it can, in fact, prove effective in limiting the virus’ spread within the body.
Ravi K. Amaravadi, the lead investigator on the trial, is an associate professor of hematology-oncology at Penn who has spent the last decade running clinical trials to see how hydroxychloroquine can help treat various types of cancer cells. Though those trials are still ongoing, he said, the particulars of their design and existing research teams made it easier to get a similar trial for COVID-19 off the ground.
For a virus like SARS-CoV-2 to infect the body, it must reach a part of a cell called the lysosome. The acidic environment there allows the virus to emerge from its shell, which it must do to replicate and be transmitted to more cells, ultimately spreading infection throughout the body.
In previous lab testing, hydroxychloroquine and other chloroquine derivatives appear to have de-acidified the lysosome, impeding that critical step for the virus to spread. In lab tests conducted in Wuhan, China, hydroxychloroquine showed promise of slowing the spread both when applied before cells were exposed to the virus and after. Because of that, Penn’s PATCH trial will test the drug on both people who have already been diagnosed and those who are at risk of exposure.
The first study group consists of patients who have tested positive for the virus, but are well enough to quarantine at home. Statistically, most of those patients, all of whom will be seen by doctors through Penn Medicine, will get better on their own. But some will get worse and need to be hospitalized.
“Can hydroxychloroquine prevent people [from] getting worse that would get worse? In the people who might get better on their own, can it speed that up?” asked Amaravadi.
To be eligible for the study, patients must have a fever, be over 40, and have gotten sick recently.
Researchers will measure recovery using a scoring system that monitors for reduced symptoms, administered remotely by doctors and nurses. Patients will not be given another test to confirm they are negative, because of the national testing shortage.
“We’re doing clinical research in a pandemic,” said Amaravadi. “You have to use the resources that you have to make it work.”
The study will be a double-blind, placebo: Neither the 100 patients nor their doctors will know if the patient is taking a placebo or hydroxychloroquine. If the patient gets worse on the placebo side, the study may be unblinded and the patient can be offered hydroxychloroquine.
The second group of patients will be those with severe enough cases that they require hospitalization. They might be slightly older, have pre-existing conditions, or have evidence of the infection worsening via blood tests. In those patients, all of whom will be admitted at Penn’s hospitals, the researchers will offer two different doses of the medication to see if a higher dose can stabilize people faster than a lower dose.
The final group will be 200 Penn health care workers — doctors and nurses who work in emergency departments and infectious-disease wards at Penn Presbyterian and Hospital of the University of Pennsylvania. The workers will be given either hydroxychloroquine or a placebo for two months, to offer a wide window during which the worker could be exposed to the virus. The major outcome measured here will be the number of workers who contract the virus.
If the rate of infection is low enough in each sub-study, Amaravadi said, the plan is to stop the trial, publish the results, and start administering the drug to everyone.
Similar clinical trials are underway worldwide, but few have released results. In one of the few completed studies of hydroxychloroquine for COVID-19, the group that received the medication fared almost the same as the control group. In another, the results were more promising, but that test has been criticized by the scientific community for its small sample size and flawed methodology.
The Penn researchers have a collaboration with Quest Diagnostics to ensure speedy testing for study among people at home. They also have secured their own drug supply for the study from Sandoz, a unit of the pharmaceutical giant Novartis. That’s important because it means the drug won’t be coming out of an existing supply that might otherwise go to those rheumatoid arthritis or lupus patients who depend on the drug every day and are finding it in short supply.
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.




![CoronavirusPandemic_1024x512[1]](https://whyy.org/wp-content/uploads/2020/03/CoronavirusPandemic_1024x5121-300x150.jpg)