FDA shortens timing of Moderna booster to 5 months
The two-dose Moderna vaccine is open to Americans 18 and older.
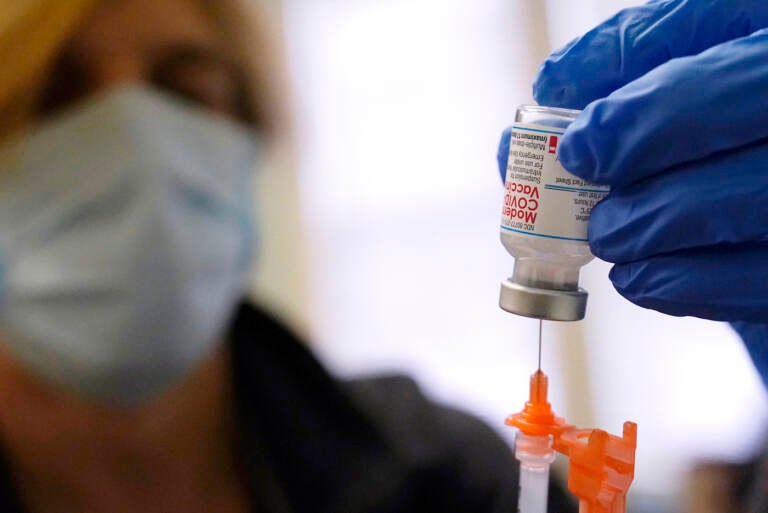
Pharmacist Kenni Clark prepares a booster dose of the Moderna COVID-19 vaccine during a vaccination clinic at City of Lawrence's "The Center," which serves seniors, families and the community, Wednesday, Dec. 29, 2021, in Lawrence, Mass. (AP Photo/Charles Krupa)
U.S. regulators on Friday shortened the time that people who received Moderna’s COVID-19 vaccine have to wait for a booster — to five months rather than six.
The two-dose Moderna vaccine is open to Americans 18 and older. The Food and Drug Administration’s decision Friday means Moderna recipients are eligible for a booster after at least five months have passed since their last shot.
That’s in line with new recommendations for recipients of the Pfizer vaccine. Initial Pfizer vaccinations are open to anyone 5 or older. But only Pfizer recipients 12 and older are eligible for boosters, and earlier this week U.S. health authorities said they can get one five months after their last shot.
In a statement, FDA vaccine chief Dr. Peter Marks called vaccination “our best defense against COVID-19” and said a shortened wait for a booster may help as the country battles a surge of the highly contagious omicron variant.
A booster after receiving the single-dose Johnson & Johnson vaccine already is urged two months later.

Saturdays just got more interesting.
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.



![CoronavirusPandemic_1024x512[1]](https://whyy.org/wp-content/uploads/2020/03/CoronavirusPandemic_1024x5121-300x150.jpg)