Canada has universal health coverage. So why is a new ‘miracle drug’ so hard to get?
Trikafta costs $360,000 a year per cystic fibrosis patient, a big price the government has hesitated to approve.
Listen 7:12
Cystic fibrosis patient Jacob Jaramillo taking his first dose of Trikafta. Trikafta costs $360,000 a year per patient, a big price the Canadian government has hesitated to approve. (Courtesy of Jacob Jaramillo)
This story is from The Pulse, a weekly health and science podcast.
Subscribe on Apple Podcasts, Spotify or wherever you get your podcasts.
33-year-old Jacob Jaramillo was born with cystic fibrosis, a severe genetic disease that causes sticky mucus to build up in his organs, especially his lungs.
When he was diagnosed, doctors weren’t sure if he would live to see 30. His family understood that if they remained in their home country of Colombia, his chances of survival would be even lower. So in 2000, when Jaramillo was 12, he and his family packed their bags and moved to Canada.
At the time, Canada was considered one of the best countries in the world for CF patients. It has universal health coverage, more access to care, and patients had better chances of getting lifesaving lung transplants.
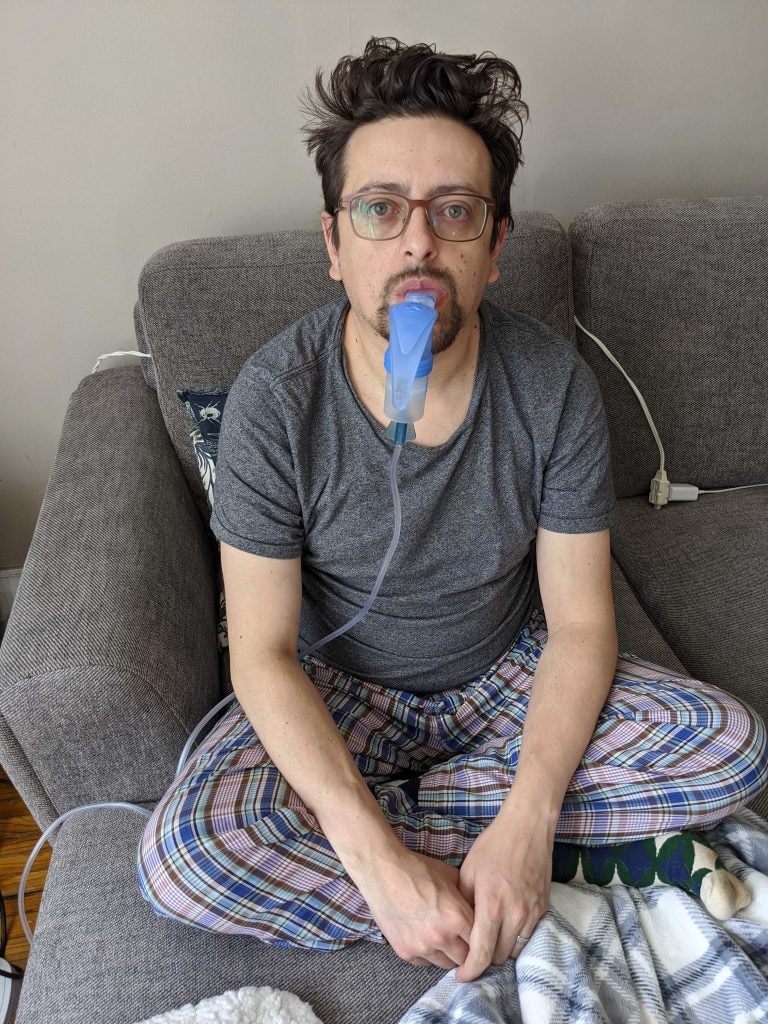
“Believe it or not, we used to be very proud that compared to our neighbors in the States, people with CF would live 10 years longer than our counterparts in the States,” Jaramillo said. “Things are clearly very different now.”
In 2019, a new miracle drug was released called Trikafta. It fixes the broken protein that causes cystic fibrosis, treating the root cause of the disease in a way that no medication has successfully done before. All patients have to do is take one pill in the morning, and one at night, every day for the rest of their lives.
However, the drug costs $360,000 a year per patient, a big price tag that the Canadian government has hesitated to approve. Christopher McCabe, a health economist who lives in Canada and studies drug pricing, said the pharmaceutical company is price-gouging.
“For expensive drugs for rare diseases, once you’ve got something licensed, you’ve got a monopoly,” McCabe said. “The company has been very reluctant to accept prices that the Canadian provinces think they can afford. And a lot of the basic science was government-funded.”
Jaramillo is angry and disappointed with how the government has handled the situation, especially after seeing Trikafta’s success in other countries over the past two years.
“It’s fair for the government to not want to pay excessive prices,” said Jaramillo. “But if the rest of the world has figured it out, then there’s no excuse. This isn’t a problem that has not been solved.”
Jaramillo’s health has been declining for years. Because cystic fibrosis affects the lungs, any kind of physical exertion became a struggle for him. Even something as simple as walking four blocks could leave him utterly exhausted. He was forced to quit his job as a chef because of the toll it took on his body.

When he first heard about Trikafta, during the Phase 3 trials for the drug, it seemed like it was what he had been waiting for his whole life. It made him reconsider what was possible.
Subscribe to The Pulse
“My wife and I, we’d love to have kids, but before Trikafta we never really talked about it,” Jaramillo said. The average life expectancy for people with CF is just 37 years old.
“I don’t wanna die when my kid is 5 years old and leave my wife with a child. So it’s more than just lung function that Trikafta has given back to our community. It’s really just hope.”
In December 2019, just two months after Trikafta was approved in the United States, Jaramillo suffered one of the worst lung infections of his life. His doctor put him on IV antibiotics, but months in his fever persisted. He had developed allergies and resistance to many of the antibiotics that had worked for him for years.

“That’s where a bit of panic sets in, because in the past things had worked for me,” said Jaramillo. “You know, it’s debilitating. I can deal with a month and crush it mentally and physically. But once you start hitting a four-month mark, it takes its toll. It takes its toll on everything physically, mentally, emotionally.”
In the meantime, the Canadian government didn’t seem anywhere close to approving Trikafta. Jaramillo was getting desperate to get the medication, but there was no way he could afford it without insurance.
He founded an advocacy organization called CF Get Loud that calls for the Canadian government to approve the medication. As luck would have it, through his advocacy work Jaramillo met someone who was able to help him.
“I met what I can only describe as a guardian angel from the U.S. who had a bit of Trikafta that he wasn’t gonna use, and he offered to send it,” Jaramillo said. “I’m like, whoa. I’m blown away, both in this sense of community and the gift itself.”
Jaramillo’s guardian angel has been sharing a prescription and sending half the dose to Canada. When Jaramillo started to take the medication, even at just a half a dose, the effects were remarkable.
“It was the best two years of my life,” Jaramillo said. “When I say that to people and I describe it as such, they’re like, ‘Are you crazy? It’s a global pandemic.’ But it legitimately has been the best two years of my life.”

But he hasn’t stopped thinking about those who still cannot get access to the medication.
“It comes with guilt,” he said. “Why me? How did I get so lucky? There’s been people that have been fighting so hard for so many years, people in worse health than I, and it’s just like, how? How did I get this gift?”
Even Jaramillo only has a limited supply, and the uncertainty makes him nervous.
“I need to ration it. I used it as a crutch to get me out of this infection. I got a taste of this amazing miracle. But my supply has been uncertain throughout,” he said. “I’m still taking a half-dose of it because I’m trying to stretch it out until we get access here in Canada.”

Jaramillo continues to push to get the drug approved. Right now, cystic fibrosis kills one person a week in Canada.
The Canadian Agency for Drugs and Technologies in Health is currently reviewing Trikafta, and it’s expected to decide whether to recommend the drug for approval at some point in June.
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.