On Radio Times: New approach to fighting pediatric leukemia approved by the FDA
Listen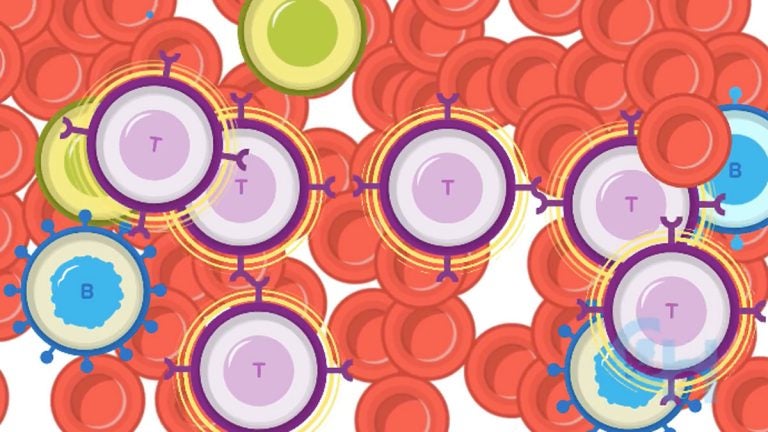
There was exciting news in the world of cancer research this week. On Wednesday, the FDA approved the first gene therapy to treat a form of pediatric leukemia. The CAR-T therapy, which was first developed at Children’s Hospital of Philadelphia and the University of Pennsylvania, is sometimes referred to as a “living drug” because it works by re-programing the patient’s immune cells to fight cancer cells.
On Friday’s Radio Times, guest host Mary Cummings-Jordan spoke with one of the lead investigators on the treatment trials, Stephan Grupp, director of the Cancer Immunotherapy Program at Children’s Hospital of Philadelphia, and with Robert Vonderheide, director of the Abramson Cancer Center at the University of Pennsylvania.
They explained that cancer is good at hiding from our T-cells, or immune cells, but the new therapy, which will be called Kymriah and manufactured by Novartis, genetically engineers a patient’s immune cells to recognize and target the cancer cells. Mary asked Grupp what happens when the immune cells go back into the body. Listen in the audio player above.
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.




