Have doubts about getting the COVID-19 vaccine? Here are some FAQs
WHYY’s readers and listeners shared their concerns. Our Health Desk Help Desk addresses them here.
Listen 5:38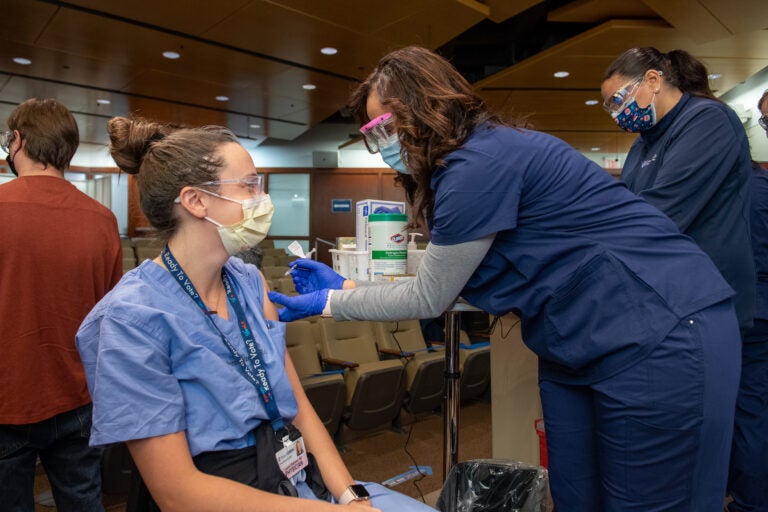
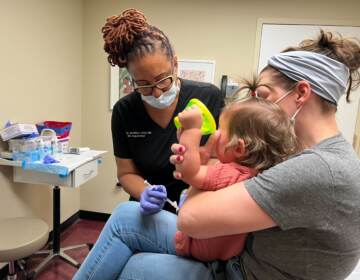
Penn Medicine frontline workers receive COVID-19 vaccinations at Pennsylvania Hospital in Philadelphia on Dec. 16, 2020. (Courtesy of Penn Medicine)
Ask us about COVID-19: What questions do you have about the current surge?
This is one of a series of articles in which reporters from WHYY’s Health Desk Help Desk answer questions about vaccines and COVID-19 submitted by you, our audience.
In May 2020, the U.S. Department of Health and Human Services launched Operation Warp Speed as an effort to support pharmaceutical companies in developing vaccine candidates for the coronavirus.
The program’s goal was to distribute safe and effective vaccines to Americans by January 2021. It succeeded.
___
This all happened so fast, and I’m worried the process was rushed.
It’s true that, until now, the fastest the United States had ever brought a vaccine through clinical trials and to market was four years — for the mumps vaccine, in 1967. But there are some key reasons that the leading SARS-Cov-2 vaccines were able to be developed so quickly.
The primary one is that though the mRNA technology used by Pfizer/BioNTech and Moderna is the first of its kind to receive Food and Drug Administration authorization for humans, it is not new.
Drew Weissman is a scientist at the University of Pennsylvania who was a pioneering developer of the mRNA vaccine technology. He said the first animal was injected with mRNA as a vaccine in the 1990s — and he has been working to refine the method ever since.
“It wasn’t just whipped up in the past couple of months,” Weissman said.
The research and development process also moved faster than usual because of the enormous investment from the U.S. government. Usually, pharmaceutical companies must commit millions of dollars to hire staff, recruiting participants for clinical trials and securing facilities to manufacture vaccines. They foot their own bills or make an argument to government agencies that the need is great enough that it’s worth chipping in.
Because the federal government’s Operation Warp Speed effort paid for much of the clinical trial research, the companies racing to develop a coronavirus vaccine didn’t have to invest as much into the development process. Even Pfizer, which did not formally participate in Operation Warp Speed, had a promise from the U.S. government that it would buy nearly $2 billion worth of its produced vaccine on the back end.
Steve Joffe, a pediatric oncologist and bioethicist at the University of Pennsylvania, sits on the data safety and monitoring board that is a part of Operation Warp Speed and has been overseeing most of the COVID-19 vaccine clinical trials that have been going on or are about to start.
“When you know you’ve got a buyer there for $2 billion of your product, I think as someone working on the business end of things, that makes you a lot more comfortable and confident investing your resources,” Joffe said.
There simply hasn’t been the same degree of investment from governments desperate to reopen their economies and save lives during other public health emergencies, he said.
“We haven’t seen anything like this pandemic and the kind of urgent demand that it created in our lifetimes.”
In fact, now that there has been such a flood of investment, leading to evidence that the mRNA delivery system can work, Weissman is hopeful that the platform will be used for other common viruses like the universal flu, HIV and malaria.
The final reason the vaccine studies were able to yield results faster than ever before is because the coronavirus is so ubiquitous. Usually, clinical trials take a long time because it’s hard for researchers to recruit enough participants to produce statistically significant results for both the placebo group and the vaccine group. But when the virus is spreading as quickly as it has been in so many parts of the world, that process can go a lot faster.
“When you’re having cases happen at the rate of thousands of cases a day, that means the vaccine can prove itself that much more quickly,” Joffe said.
In a sense, it’s a true silver lining of the raging pandemic: More infections mean faster research.
Some groups of people weren’t included in the clinical trials. Is it safe for them to get the vaccine? Should people who are pregnant or breastfeeding get the vaccine?
The FDA granted emergency use authorization for the Moderna vaccine in anyone age 18 or older. Emergency use authorization for the Pfizer vaccine was given for anyone age 16 or older.
Both trials excluded those who are pregnant, so the data there comes from people who got pregnant inadvertently during a vaccine trial. That means we don’t have enough data to say for sure how the vaccines could affect pregnancy.
However, both the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine say those who are pregnant or breastfeeding should be given access to the vaccine.
Laura Riley is the chair of obstetrics and gynecology at Weill Cornell Medicine in New York and chair of the immunization committee at the American College of Obstetricians and Gynecologists. She said it would be nice to have more data on pregnancy and the vaccines, but there is nothing to suggest they are unsafe.
“Biologically, we don’t think it’s plausible … that there’s any reason to be concerned about moms’ safety or fetuses’ safety,” Riley said.
She explained that all the activity from the mRNA vaccine happens within the body of the person who gets vaccinated and will not get into the cells of a placenta. She added that the activity from the vaccine will not get into sperm or eggs either, so it should not affect people who want to have children.
Ultimately, Riley said, it all comes down to weighing the risks versus the benefits of getting a COVID-19 vaccine. The Pfizer and Moderna vaccines are very effective, according to the clinical trial data.
People who are pregnant should take the vaccine if they are part of a group that is prioritized for one, like health care workers, and they are offered one, said Vincenzo Berghella, director of maternal fetal medicine at Thomas Jefferson University. Most pregnant individuals who get COVID-19 have mild or no symptoms, he said, and his team has treated almost 200 pregnant women with COVID by now. But the research shows that pregnant people who get COVID-19 are more likely to have serious outcomes, like needing intensive care or a ventilator.
Both Pfizer and Moderna said they plan to specifically study what happens to pregnant people who get the vaccine going forward. There are ongoing studies of what happens to those who get COVID-19. Some researchers are asking breastfeeding mothers who get the vaccine to donate their breast milk for studies, and that includes Thomas Jefferson University in Philadelphia, said Berghella.
How about kids?
Neither the Moderna nor the Pfizer/BioNTech trials included people under 16, which Joffe said makes sense, because you want to have some information about a vaccine’s safety before enrolling kids in trials. Children can’t give their own informed consent, or permission to participate, the way adults can. Since children have not become as ill from the coronavirus as adults, there is less risk in waiting to enroll them. Still, the goal is to have a vaccine approved for kids eventually.
“It’s a balance,” Joffe said. “Safety and wanting to protect against risk on the one hand, needing pediatric data on the other. It’s a really difficult dilemma.”
Trials have already begun for young people ages 12 to 16. These studies will likely be less focused on efficacy since it’s clear the vaccine works to prevent illness, and more on safety in those groups.
Is the vaccine recommended for people who already had COVID-19?
If someone has already gotten COVID-19 and recovered from it, they would have natural immunity, but it’s not clear how long that lasts. Some preliminary research suggests it could be months. Although it is rare, people can get reinfected.
Scientists say that it’s not a bad idea for those who already had the virus to get vaccinated — whether they had symptoms or not — and that testing positive in the past should not be used as a reason to deny someone the vaccine.
In its recommendations for the Pfizer/BioNTech vaccine, the Advisory Committee on Immunization Practices noted that data from the trials suggest the vaccine is safe and likely effective in people who were previously infected.
I care for an elderly parent. If I get the vaccine, could I still pass the infection on to her?
That gets at the heart of one of the most pressing questions left unanswered by the trials. Weissman explained that while both trials concluded the vaccines are very effective at preventing the person who is vaccinated from getting sick with COVID-19, they didn’t test for whether the vaccine stops people from becoming infected and transmitting the disease. He said coming research will “help us determine whether the vaccine is an absolute stop, or we still have to be careful.”
I understand that it’s impossible for the vaccine to give me COVID-19, but I have a serious lung and heart condition and worry that even the side effects of the vaccine could make them worse.
The trials included a range of participants, including older people and those with a range of chronic health conditions. In Pfizer’s trial, nearly half the people who received the vaccine had a medical condition that would increase the risk of severe cases of COVID-19, such as chronic lung disease, heart conditions, diabetes and obesity. In Moderna’s trial, 22% did.
“We have from the trials data on thousands of people who had complicated serious medical conditions, and we have not seen evidence from the trials that there were particular health risks to people who had chronic medical conditions,” Joffe said.
What’s in the vaccine? Can it alter my DNA?
The mRNA vaccines (the technology used by Pfizer/BioNTech and Moderna) is made of two parts: the messenger RNA, which carries a sort of instruction manual that prompts an immune response in the body; and lipid nanoparticles, which are the delivery vehicle for the mRNA.
The mRNA vaccine enters the cells and teaches them how to produce the “spike” protein that naturally occurs on the coronavirus. Those instructions — the mRNA itself — disintegrate over time; they don’t hang around in your body. Your body detects the proteins built by your cells as a foreign object, triggering an immune response. To fend off the invader, your body creates antibodies, just as it would if you contracted the coronavirus, to protect you from those proteins. Your body also breaks down the proteins that sparked the response — they don’t remain in your cells either.
None of that process happens in the nucleus or penetrates it, which it would need to have any sort of impact on DNA.
“The RNA does not interact with DNA, cannot alter the DNA in any way,” Weissman said.
The lipid nanoparticles — the second part of the mRNA — are there to protect the RNA from degrading before they enter the body. They are the reason that the vaccines need to be kept at cold temperatures. They help the mRNA effectively enter the cells and, by some luck, turn out to also help a second tier of cells develop protection against the protein, which may mean immunity will last longer. Lipid nanoparticles are well tolerated by humans, said Weissman, and already exist in FDA-approved drugs at 50 times the dosage as in this vaccine.
What about the long-term effects the vaccine could have on my body?
There is no way to know the long-term impact that an mRNA COVID-19 vaccine could have on the body, since it hasn’t existed for very long. But we can look for clues in other, similar vaccines.
Two Phase One clinical trials, started in 2015 and 2016, used mRNA technology. They were small, enrolling about 50 patients in each. They reported results in 2018, and there have been no long-term adverse events noted in either of those trials.
Based on that, and the fact that side effects from vaccines in general usually happen soon after the shot and not years later, researchers say they have no reason to believe that there would be any long-term damage.
Ultimately, it comes down to a risk calculation of which could be worse: the chance of what seems to be a safe and effective vaccine, or the potential impact of getting COVID-19.
“Are you gonna wait 30 years? If you get coronavirus before 30 years, you might not survive it,” said Dr. Ala Stanford, head of Philadelphia’s Black Doctors COVID-19 Consortium.
“Whatever we know about the risks or side effects of the vaccine — they seem to be kind of miserable for many people, but short-lived — they are far and away less worrisome than the complications of the coronavirus itself,” he said.

Get daily updates from WHYY News!
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.



![CoronavirusPandemic_1024x512[1]](https://whyy.org/wp-content/uploads/2020/03/CoronavirusPandemic_1024x5121-300x150.jpg)