How can we tweak the vaccines?
Add more boosters? Redesign to anticipate new variants? Researchers are evaluating possibilities for improvement.
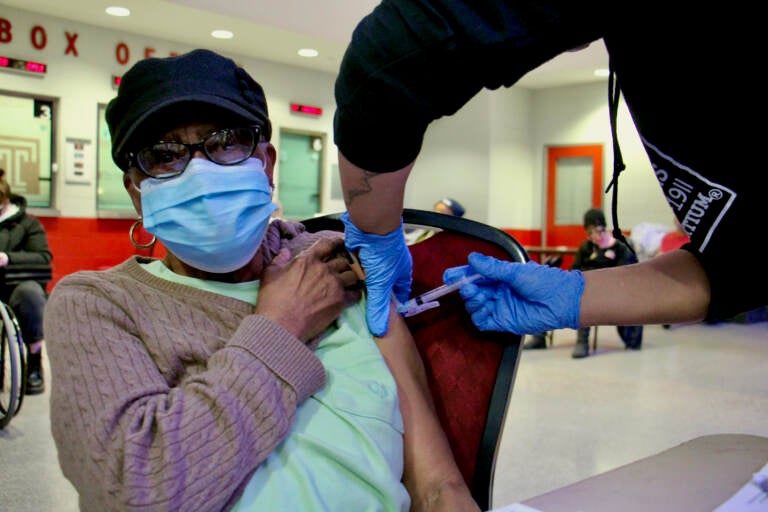
Edna Evans, 88, gets a COVID-19 vaccination at the Liacouras Center at a vaccine clinic organized by the Black Doctors Consortium. (Emma Lee/WHYY)
This is one of a series of articles in which reporters from WHYY’s Health Desk Help Desk answer questions about vaccines and COVID-19 submitted by you, our audience.
The vaccine boosters made by Pfizer and Moderna provide strong protection against serious illness from COVID-19 and hospitalization. But their protection does wane over time, according to a Centers for Disease Control and Prevention study published earlier this month.
Here’s what we know about what that means for us.
What does the CDC study show?
Effectiveness after a third dose is stronger than after the second dose of the vaccine, but it diminishes over time. Researchers found that with the omicron variant of the coronavirus, a third shot was 91% effective in preventing hospitalization for two months, but fell to 78% after four months. The booster was 87% effective in preventing trips to urgent care and the emergency department for two months, but fell to 66% after four months.
By comparison, the study found that when omicron was predominant, just two vaccine doses were 71% effective against hospitalization for two months; that decreased to 54% by at least five months.
The study did not compare waning immunity by age or health condition, however.
“The new data on boosters suggests that the third dose will boost protection for some period of time, but that protection also will go away,” said Dr. John Wherry, director of the Institute for Immunology at the Perelman School of Medicine at the University of Pennsylvania. “We know that after vaccination or infection you get a peak in antibodies. They go up quickly, they reach a much higher level, and then they sort of decay back down to a, we’ll call it, steady state.”
Wherry said it’s important to know that there are two types of protection. Protection from severe disease, hospitalization, and death is the ultimate goal of any vaccine, he said. Protection from infection is much more difficult to achieve, and is somewhat less important in terms of overall public health, he said.
“What we really need to do is protect people from ending up in the hospital and dying. We need to protect our health care system from having so many COVID patients in the hospital that we can’t provide other kinds of care. And we need to get to the point where when there are infections in the community, we’re not putting other people at risk so we can keep our schools and businesses open,” Wherry said.
What waning immunity from the vaccines means for each of us will depend on whether new variants arise and how serious they are, as well as our individual risk factors, said Martin Blaser, director of the Center for Advanced Biotechnology and Medicine at Rutgers University. Going forward, older people or those with certain health conditions might have to be more wary, he said.
“They may wear a mask more often. They may avoid gatherings more often. They may want to get vaccinated as often as is necessary,” Blaser said.
What could this study mean for future variants?
The virus can always change, Wherry said, so scientists need to be prepared for future variants. But he said it’s promising that a third shot still performs reasonably well against both delta and omicron. Though it’s possible that antibodies will fade over time, T-cells — another part of the immune system — are much less affected and can still be resilient to variants, Wherry added.
There are many directions the pandemic could go, Blaser said: There could be a period when new variants don’t circulate much at all; a new variant could arise resembling omicron; a new variant could resemble the original strain; or a new variant could arise that resemble neither.
Will I need a fourth shot?
About 91 million people in the United States have received boosters, and close to 8 million got them at least four months ago.
Dr. Anthony Fauci, chief medical adviser to President Joe Biden and the director of the National Institute of Allergy and Infectious Diseases, said that decisions around boosters will be based on factors such as whether hospitalizations among boosted people increases. It’s possible people might need another shot, but it might also depend on a person’s age or underlying health condition, he said.
Wherry said there’s a possibility that you may need another booster in the future. How often you’ll need one is another question.
“I think it’s important to state that we need to be prepared to react to any big changes or surprises that this virus throws at us. Saying that we’ll never need another booster is kind of irresponsible, and we need to be more humble about that,” Wherry said. “If in May we see an omicron-like virus show up somewhere in the world, we have to reevaluate everything and be ready to do that both scientifically and also societally.”
“But I think most of the science says that boosting every four months is a) not immunologically all that valuable, and b) just not sustainable,” he said.
Some people, such as those who are immunocompromised, might need boosters more frequently, Wherry said. But for the general public, he said, boosters every four months are unlikely.
What’s the alternative to boosters every four months?
The vaccine can be changed quickly, effectively, and safely, Wherry said. It was only 66 days from the time that researchers had the sequence of the virus that causes COVID-19 to the day the first person was injected with a vaccine in the first clinical trial.
“That means that we can react really quickly to changing information and tweak the vaccines. The question is how do we tweak them? And what’s going to be beneficial?” Wherry said.
Omicron-specific vaccines are being researched now. Wherry argued that those vaccines may not be necessary because the original works pretty well against omicron, but that they should be on the shelf just in case.
A team at the University of Pennsylvania is working to develop a universal vaccine that would protect people from all variants and give them broader immunity.
“Whether that will be successful or not, I don’t know. But certainly people are looking for something that will be much more generic,” Blaser said. “You have a measles vaccine, it lasts for years and years; if you have a shingles vaccine, it lasts for years and years. But the influenza vaccine basically lasts for one season, and these COVID vaccines seem to be lasting even shorter.
“Right now, the big wave of omicron is receding — omicron is going away almost as fast as it came, which is actually, I think, very good news. And right now on the horizon, there’s nothing to replace it. Now, that could change tomorrow, or it could change six months from now. I don’t know the answer,” Blaser said.
A yearly COVID-19 shot would be very reasonable for the general population, he said.
“People are very accustomed to taking it for the flu, and because of the severity of COVID, people, I think, will line up to take this one,” Blaser said. “If they have to do it every six months, it won’t be quite as good, but there’ll be some people who won’t do it. And if it has to be every four months, there’ll be even fewer people who want to do it.”
Researchers can make a new mRNA vaccine and start clinical trials within about 100 days, Pfizer’s CEO has said. Trials would take another three to four months.
“We’re really talking about probably a turnaround time of around six months, when we have everything working properly, to get both a safety signal and an efficacy signal from a trial and then decide what to do next,” Wherry said. “We need financial resources, we need organization resources to do that at scale, and we need the administrative backing from the standpoint of approvals, and regulation, and safety to make sure we’re doing things as efficiently as possible.”

Saturdays just got more interesting.
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.





![CoronavirusPandemic_1024x512[1]](https://whyy.org/wp-content/uploads/2020/03/CoronavirusPandemic_1024x5121-300x150.jpg)