Coronavirus vaccine developers issue joint pledge on safety, standards
The announcement comes amid worries that the FDA will be under political pressure to approve a vaccine before tests to prove it is safe and effective are finished.
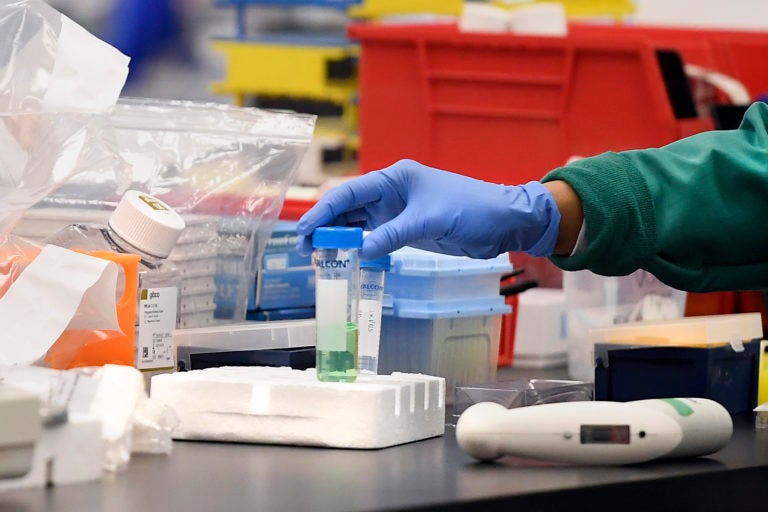
A researcher moves a vial in a lab. Labs are ramping up their research, in the hopes of finding a vaccine for COVID-19. (Jessica Hill/AP Photo)
The top executives of nine drugmakers likely to produce the first vaccines against the novel coronavirus signed an unprecedented pledge meant to boost public confidence in any approved vaccines.
The companies said Tuesday that they will stick to the highest ethical and scientific standards in testing and manufacturing and will make the well-being of those getting vaccinated their top priority.
The announcement comes amid worries that the U.S. Food and Drug Administration will be under political pressure to approve a vaccine before tests to prove it is safe and effective are finished.
Meanwhile, public health officials worry that if many Americans don’t get the vaccine, it will be harder to reduce the spread of COVID-19.
The pledge was signed by the chief executive officers of American drugmakers Johnson & Johnson, Merck, Moderna, Novavax and Pfizer, and European companies AstraZeneca, BioNTech, GlaxoSmithKline and Sanofi. BioNTech has partnered with Pfizer on one of the vaccines now in the final round of human testing.
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.


![CoronavirusPandemic_1024x512[1]](https://whyy.org/wp-content/uploads/2020/03/CoronavirusPandemic_1024x5121-300x150.jpg)