Phase I study finds ‘gene editing’ aimed at making patients’ cells resistant to HIV to be safe
Listen-

-

-

-

-

-

Laboratory Technician Anisha Chirmule pulls growing cells out of an incubator at the Clinical Cell and Vaccine Production Facility at the University of Pennsylvania. (Kimberly Paynter/WHYY)
-
-
-

-
For decades, HIV has outpaced the scientists trying to eradicate it, often morphing and hiding deep in patients’ bodies. Researchers at the University of Pennsylvania recently tested a gene-editing technique with hopes that, one day, such an approach might help patients get rid of the virus altogether.
For decades, the HIV virus has been outpacing scientists trying to eradicate it, often morphing and hiding deep in patients’ bodies. Researchers at the University of Pennsylvania recently tested a new gene-editing technique in patients, with hopes that one day such an approach might help patients rid the virus altogether.
The pilot, published in the New England Journal of Medicine, found that editing a specific gene is even possible in humans. The modified cells also showed signs of living longer in the presence of HIV.
For Dr. Pablo Tebas, a lead on the study, it marks a positive step on a long path aimed at figuring out how to eliminate patients’ need for HIV drugs.
“That’s the holy grail that we are pushing toward for the next, many years,” says Tebas.
Researchers focused on other diseases, like hemophilia and bubble-boy disease, also have an eye on the emerging gene-therapy approaches.
Research built on natural immunity concepts
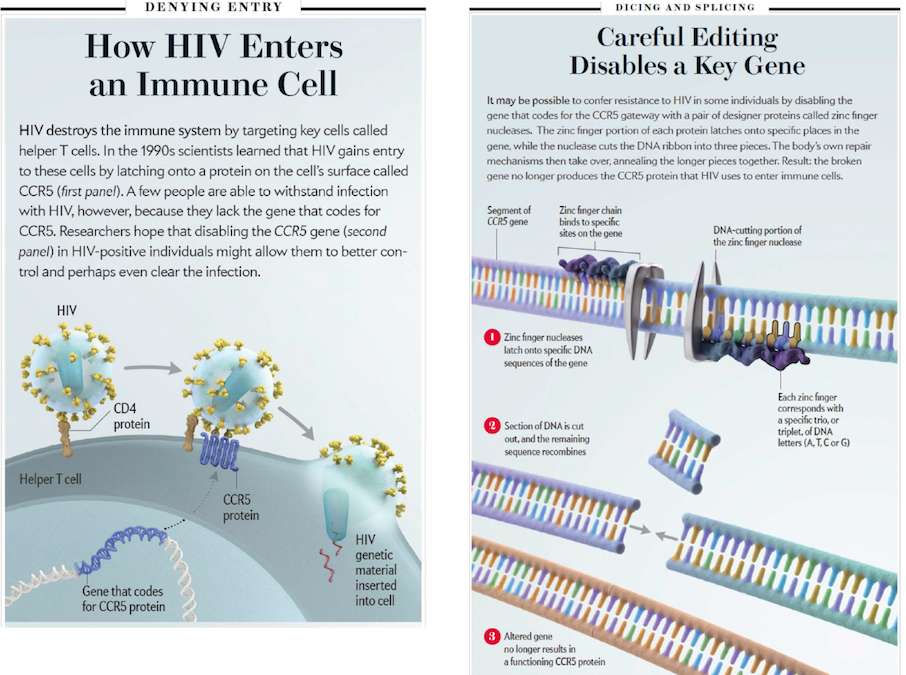
CCR5 is a molecule that holds a lot of significance to HIV researchers.
For people born without CCR5 genes, they’re generally resistant to the most common strain of HIV. Doctors have long known some people carry this natural immunity, but it wasn’t until the 90s that they pinned down this lack of CCR5 as the source of protection.
“Recognizing that the virus needed this molecule, CCR5, and that humans could live without it, ok that was a breakthrough,” says Dr. James Hoxie, director of Penn’s Center for AIDS Research and a longtime Philadelphia doctor who’s been in the thick of HIV since the virus first surfaced here.
The mutation requires that both gene copies in a person’s DNA, coming from mom and dad, are missing CCR5. Individuals with just one missing copy are not immune to the common HIV strain, but seem to do better at controlling the virus.
Hoxie, who happens to fall in that one percent of people who carry this mutation (and was once profiled by Pulse contributor Kerry Grens), says the discovery of CCR5’s role led to a new drug, and more than that, created the possibility for even thinking about genetic approaches to making cells resistant to HIV without drugs.
Building on this concept, some of Hoxie’s colleagues, including Carl June, Pablo Tebas and Bruce Levine, have been trying to modify cells of HIV patients, to act like those of individuals like Hoxie, in resisting the virus.
Twelve patients studied
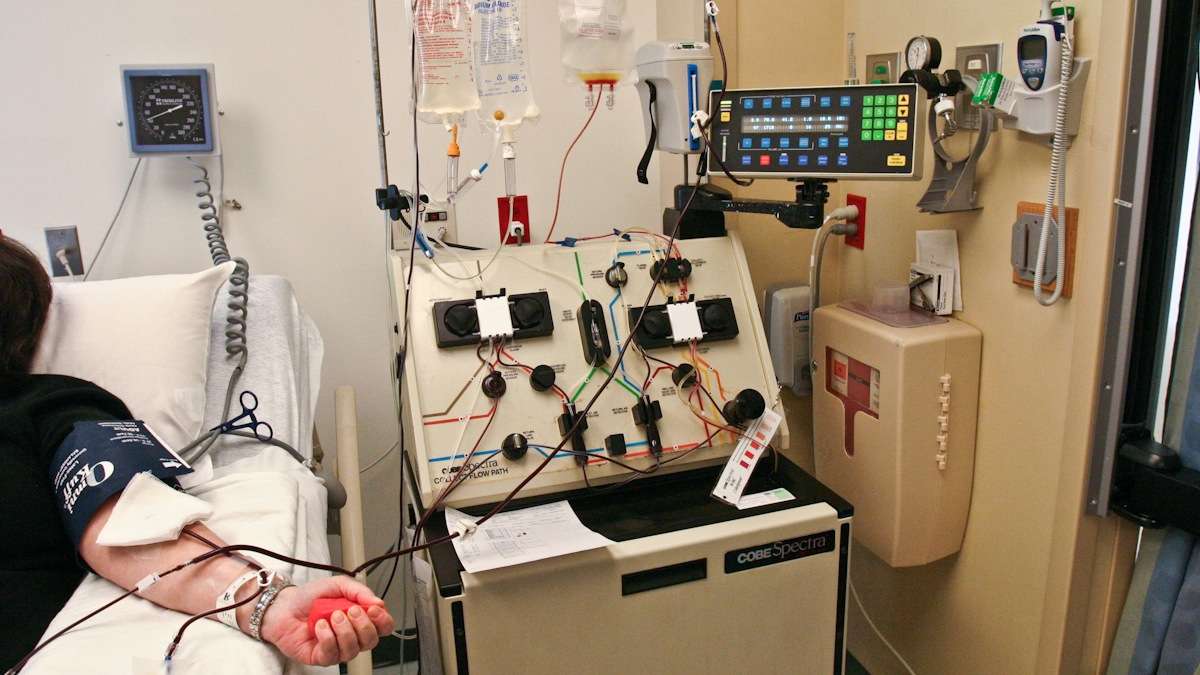
In 2009, Researchers at Penn recruited 12 patients, all had HIV for some time and were healthy, thanks to ongoing therapies. They drew blood samples and then isolated patients’ T cells. Those are the so-called brains of the immune system, which the AIDS virus attacks.
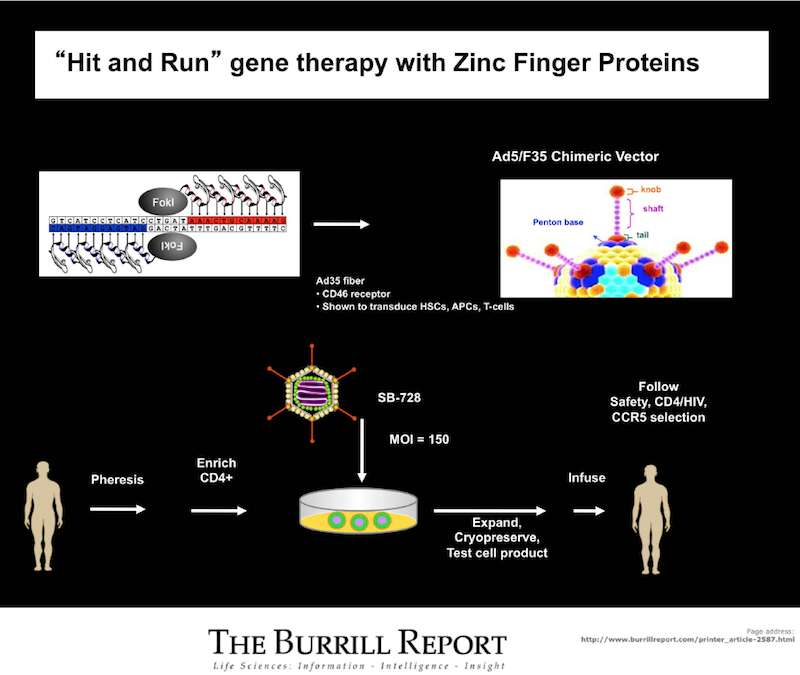
They then administered a technique, developed by California-based Sangamo Biosciences, which uses a natural protein agent called Zinc Finger Nucleases [ZFN], engineered to target and then cut out that CCR5 gene, which HIV first depends on to get into cells and take over.
“The precision of this required is really astonishing,” says June. “There are about a billion of these molecular codes in our DNA and that needle in a haystack was actually surgically excised here and the rest left intact.”
Being able to manipulate T cells is something that June and his colleague, Bruce Levine, have been working on for over a decade, but the part which hasn’t been done before is the actual gene editing.
“You can think of this as a molecular scissors to specifically go to one gene and knock that out and prevent that protein from being expressed from the surface of a cell, meaning HIV is not able to enter cells,” says Levine.
In the lab, Levine received the white blood cells of those 12 HIV patients and applied the ZFN. The process involved incubating cells with ZFN for about 10 days. Dr. Pablo Tebas has another way of looking at ZFN’s role removing CCR5.
“It’s like closing one door that the virus uses to enter the cell by removing it,” says Dr. Tebas, who oversaw the patient side.
Once the cells were modified, with the CCR5 gene disabled in 11 to 28 percent of those treated, Tebas then matched them back up with the 12 patients and re-injected them.
Then half of those patients stopped their normal HIV treatment for up to three months, to see if those genetically modified cells had a better chance of surviving in the presence of HIV.
This was a phase I, nonrandomized, uncontrolled study. The main takeaway: researchers were able to do the procedure at all, and it was safe. It was reported that one patient experienced a serious adverse reaction to the reinfusion process, and had to go to the emergency room.
Doing ‘just about anything’ to help find a cure
One of the patients taking part in the study was 53-year-old Jay Johnson. Diagnosed with HIV in 1991, Johnson says the virus has been undetectable in his body for nearly 10 years, thanks to antiretroviral therapies, making him an ideal fit for the study. Johnson, a nurse by trade and volunteer coordinator at ActionAIDS, was excited to participate.
“I would do just about anything to try to find a cure for this,” says Johnson, adding that he doesn’t believe he’s at risk for serious side effects. “If it’s going to help someone, then I’m on board, hopefully it will be something to help me, but if it can help the future, then I’m all for it.”
He describes experiencing severe chills, cramping and spasms minutes after his treated cells were reinfused, symptoms he was told are consistent with a transfusion reaction and a sign his body was absorbing the cells.
Johnson says ever since an HIV patient, the “Berlin patient,” became HIV-free after a bone marrow transplant for Leukemia a few years ago, he has a renewed hope there might one day be a cure for AIDS. Scientists are still trying to figure out how that happened. The patient did get the transplant from a donor who had the CCR5 mutation, leading some to wonder whether that might have played a role in eradicating the virus.
Researchers are both hopeful and cautious. HIV is tricky. Last winter, the virus made a comeback in two patients in Boston, who were thought to be HIV free after a bone marrow transplant. But Tebas and others say ‘successes’ and ‘failures’ alike are providing more clues about how HIV works and how it hides, leading to a more comprehensive understanding of the virus and possible new ways to combat it.
Tebas, meanwhile, says in closely monitoring Johnson and the other 11 patients, he found those modified cells did show signs of doing well in the body.
One unexpected observation, the virus was completely undetectable in one of the patients (not Johnson) during the time meds were stopped.
“It was incredible,” says Tebas. “When you live the moment you don’t think too much about it, and then when you look back you realize that was the highlight.”
Turns out, some of the cells in that patient already naturally lacked that CCR5 gene, key for HIV.
More research needed
At its core, this was a safety study to see if the procedure could even be done in patients. To really determine the actual effectiveness of the approach down the road, more studies are needed.
It’s a point Dr. Mark Kay emphasizes. He’s a professor of pediatrics and genetics at Stanford University. He wasn’t involved in the study but wrote an accompanying editorial in the New England Journal of Medicine. Kay says this gene-editing concept may have applications beyond HIV, but the technology isn’t perfect, and it will be important to keep an eye on patients’ health over time.
“While we genetically modify and create these enzymes that can recognize these specific DNA sequences, like anything, it’s not absolutely perfect. There are some events and breakages that may occur in other sequences that may be close to but not exactly the sequence, and this is called an off targeting effect, or as with many different types medications we take, can create a side effect,” says Kay. “And that question remains is how substantial will these off targeting effects be in the long term of these individuals.”
So far, off targeting side effects don’t appear to be of great concern with the ZFN process. Kay says another question moving forward will be whether the efficiency of the treatment can be increased, to knock out more copies of CCR5 to produce cells totally deficient of the gene.
Tebas, meanwhile, is continuing to monitor patients and will be for years. He says they’re doing well, and plans to expand the study this spring.
The study’s funding came from the National Institute of Allergy and Infectious Diseases, Penn and Sangamo Biosciences.
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.



