Side effects, transmission, efficacy: What do we know about the COVID-19 vaccine now?
WHYY’s Help Desk Health Desk looked into how ongoing research and months of real world distribution has changed what we know about the vaccine.
Listen 5:36
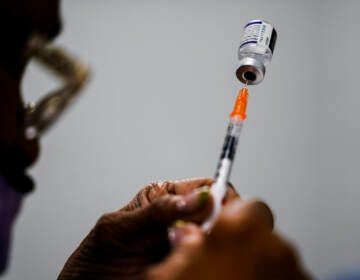
Dr. Christophe DeBrady of the Black Doctors COVID-19 Consortium prepares doses of vaccine for the hundreds who turned out for a mass vaccine clinic at the Liacouras Center at Temple University. (Emma Lee/WHYY)
This is one of a series of articles in which reporters from WHYY’s Health Desk Help Desk answer questions about vaccines and COVID-19 submitted by you, our audience.
Nearly 60 million COVID-19 vaccine doses have been administered across the country since they were authorized in December. This week, WHYY’s Health Desk Help Desk looked into how ongoing research and months of real world application has changed what we know about the vaccine.
One listener, Luz Pagan from North Philadelphia, is getting her vaccine soon, but is worried because she says she heard the vaccine isn’t fully FDA approved. She’s concerned that as a diabetic, she might have unexpected side effects. What do we know about side effects now? Should she be concerned?
First things first: the vaccine is authorized for emergency use by the FDA. That’s technically not the same thing as approval, but that’s mostly because the official FDA vaccine approval process can take a while… up to several years! Especially in a global pandemic, it makes sense to speed up the process by using emergency authorization to help save lives. So far, all research on the vaccine indicates it’s quite safe and incredibly effective.
Initial trials for both the Pfizer and Moderna vaccines included people with diabetes, as well as people with hypertension or obesity. Those trials indicate that the vaccines worked for all of those groups, and there was no sign of irregularities or different side effects for these specific conditions. In fact, researchers recommend it’s a good idea for people with diabetes to get vaccinated because they’re at a greater risk for more severe cases of COVID-19.
And while they may be uncomfortable, side effects are actually a good sign. Medical experts say they’re pretty normal and mean your immune system is working and building protection.
“The side effects to date are all just based on your immune response being activated and responding to this vaccine, which is a good thing,” said Dr. Paul Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia and a professor of pediatrics at the Perelman School of Medicine at Penn. “Products of your immune system…can cause symptoms like fever or headache or muscle pain, joint pain or fatigue. That’s true, but it usually only lasts a day or two and then it goes away.”
The CDC said the most common side effects are fever, chills, headaches, and exhaustion, in addition to pain or swelling in the arm where you got the shot — and again, they should go away after a couple of days. So if you get them, you shouldn’t be concerned.
Another listener, Roxanne Burt, just received both the first and second doses of the vaccine through her job; she’s in her 60s and says she didn’t have any side effects at all. Does that lack of immune response mean she’s not actually protected?
The short answer is: not at all! Some of the people inoculated in the vaccine trials had severe side effects, while others had mild ones, and most didn’t report any side effects at all. But in all cases, they were still protected after receiving the shot.
The Pfizer and Moderna vaccines train your immune system to identify and fight off a particular viral protein. That’s why side effects tend to be worse after the second shot. The first shot teaches your body how to develop the antibodies to defend itself, and by the time the second shot is administered, your immune system is armed and ready to kick into action.
So far, we’ve seen more intense side effects for younger people than older people, possibly because their immune systems are more robust. But even if you don’t feel those side effects, your body is still developing defenses.
When the vaccine first came out, scientists said we didn’t know yet whether it would prevent transmission, or just stop you from getting sick. Have we learned any more about this now? After I’m vaccinated, could I still spread the virus?
Multiple early research studies seem to point us toward the idea that vaccines could partly reduce transmission, even if it doesn’t completely eliminate the possibility. That said, the CDC is still advising that vaccinated people act as if they could transmit the virus: washing their hands, wearing a mask, avoiding indoor spaces with other people, etc.
Offit said that caution is because we still don’t know for sure.
“The two studies by Pfizer and Moderna were done to see whether or not the vaccine prevented against disease — not whether it prevented asymptomatic infection [or whether] you could still be contagious,” he said. “[But] as a general rule, people who are vaccinated shed less virus…than those who weren’t vaccinated.”
In other words, people who get vaccinated and become infected will still carry less virus in their bodies than unvaccinated people who get infected. The less virus a person has in their body, the less likely that person is to pass it on to someone.
Several listeners also had questions about vaccine effectiveness and clinical trial reports. What do companies mean when they say their vaccines are 95% effective?
First of all: Effectiveness and efficacy aren’t exactly the same thing. A vaccine’s efficacy tells us how well it worked to prevent disease in people in clinical trials, while its effectiveness tells us how well it works when given to people in real-world conditions.
For example, the Pfizer vaccine has 95% efficacy. That doesn’t mean that only 95 out of every 100 people vaccinated are protected and an unlucky five are not. Instead, efficacy measures relative risk. It means that 95% of the people who got COVID-19 during the observed trial were those who hadn’t received the vaccine. That indicates that the people who were vaccinated had a 95% lower risk of getting COVID than people in the control group who didn’t get the immunization.
Perhaps more importantly, the small group who were vaccinated and got COVID mostly had mild cases; they weren’t hospitalized, and none of them died. So the vaccine, which aims to prevent severe sickness and death, is ultimately successful. The same is true for vaccines that haven’t hit the market in the United States yet, which have lower efficacy rates: 66% for Johnson & Johnson. While these efficacy rates look much lower on paper than Pfizer and Moderna, no one in the vaccine groups for these trials became severely ill or died; all three vaccines are highly effective.
Finally, neither measure — efficacy or effectiveness — is an accurate or useful way to tell how likely you, personally, are to get sick. That’s because your personal risk is based on a variety of factors: the amount of exposure you have to the virus, the strength of your immune system, your age or underlying health conditions, and more. That’s also why most experts are saying you shouldn’t focus too much on efficacy percentages; instead you should just try to get whatever vaccine is authorized and available.
That’s Offit’s take, anyway.
“The goal of this vaccine is to keep you out of the hospital, keep you out of the intensive care unit, keep you out of the morgue,” he said.

Get daily updates from WHYY News!
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.




![CoronavirusPandemic_1024x512[1]](https://whyy.org/wp-content/uploads/2020/03/CoronavirusPandemic_1024x5121-300x150.jpg)