Researchers confront a ‘line-in-the-sand’ moment for drug-resistant bacteria
Globally, antibiotic-resistant bacteria are becoming an increasing problem. The Wistar Institute convened researchers to discuss alternative treatments.

Gram negative bacteria such as salmonella, Escherichia coli (E coli), yersinia pestis (plague)(Wistar Institute)
It’s Ami Patel’s job to worry about infections most people don’t regularly think about:
Klebsiella pneumoniae. Neisseria gonorrhoeae. E. coli. Salmonella. Pseudomonas.
Those are all bacteria that, in recent years, have developed resistance to traditional antibiotics. As a researcher at the Wistar Institute in Philadelphia, Patel is investigating new ways to combat them.
“I think we have seen this coming for a very long time, and over the decades researchers have been trying to get around this problem,” Patel said. “Unfortunately, bacteria are evolving at the same time as researchers are trying to evolve their strategies. So every time a new antibiotic or a new variation of an antibiotic comes out, the bacteria quickly adapt soon afterwards. And so what we’ve developed, suddenly it doesn’t work anymore.”
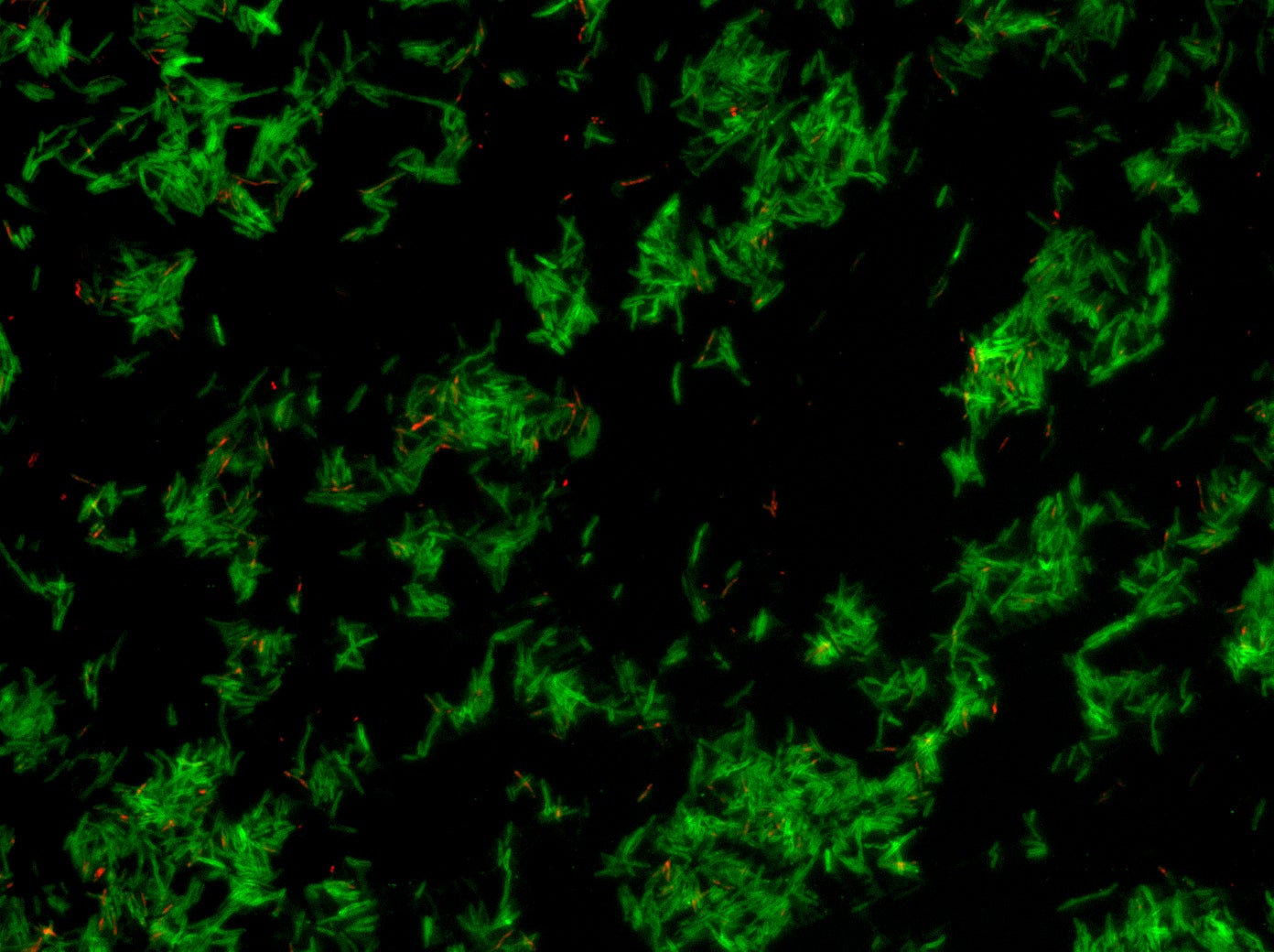
Numerous bacterial infections have developed antibiotic-resistant strains in recent years — urinary tract infections, tuberculosis, gonorrhea, pneumonia. That has alarmed researchers and physicians, who worry they are losing valuable weapons in their medical arsenal.
Antibiotics have been effective tools in the medical toolbox for decades, ever since penicillin became commercially available in the 1940s. But the rise of antibiotic resistance has made that toolbox less reliable, and many of the drugs doctors counted on to treat patients aren’t working anymore.
“There have been no new antibiotic-resistant [focused] classes of drugs developed since the 1980s,” said David Weiner, executive vice president at the Wistar Institute.
So, he said, for the last few decades, doctors have been using antibiotics already on the market, while scientists have been modifying them.
“Those modifications, there’s concern, are not really going to hold the tide, and we need new approaches to prevent this enormous growth in antibiotic-resistant infections,” he said.
That’s why this week scientists including Patel and Weiner convened at a symposium at the Wistar Institute, where experts from across the country discussed new treatments and approaches to treating antibiotic-resistant bacteria and how scientists can combat them.
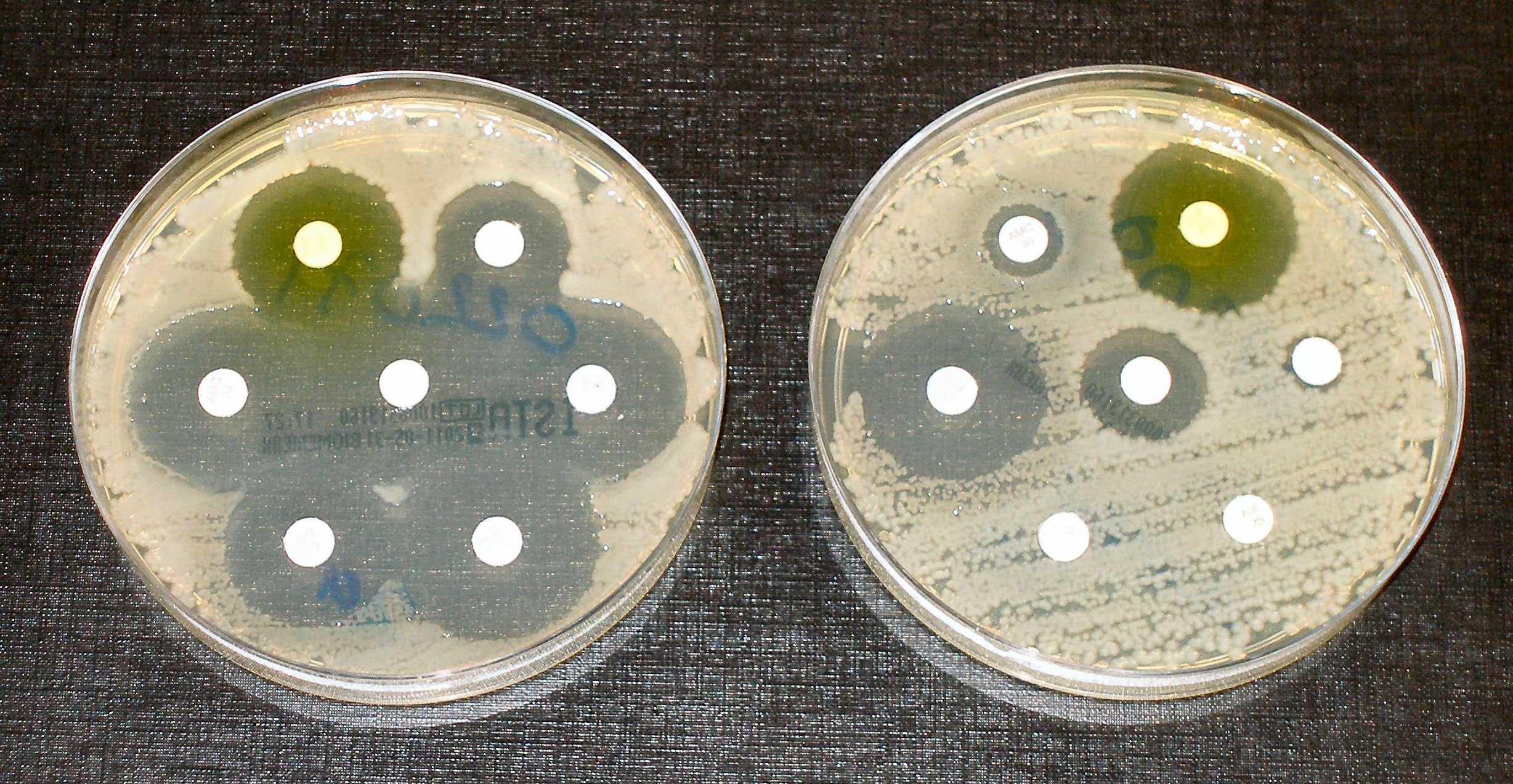
Routine procedures could become risky
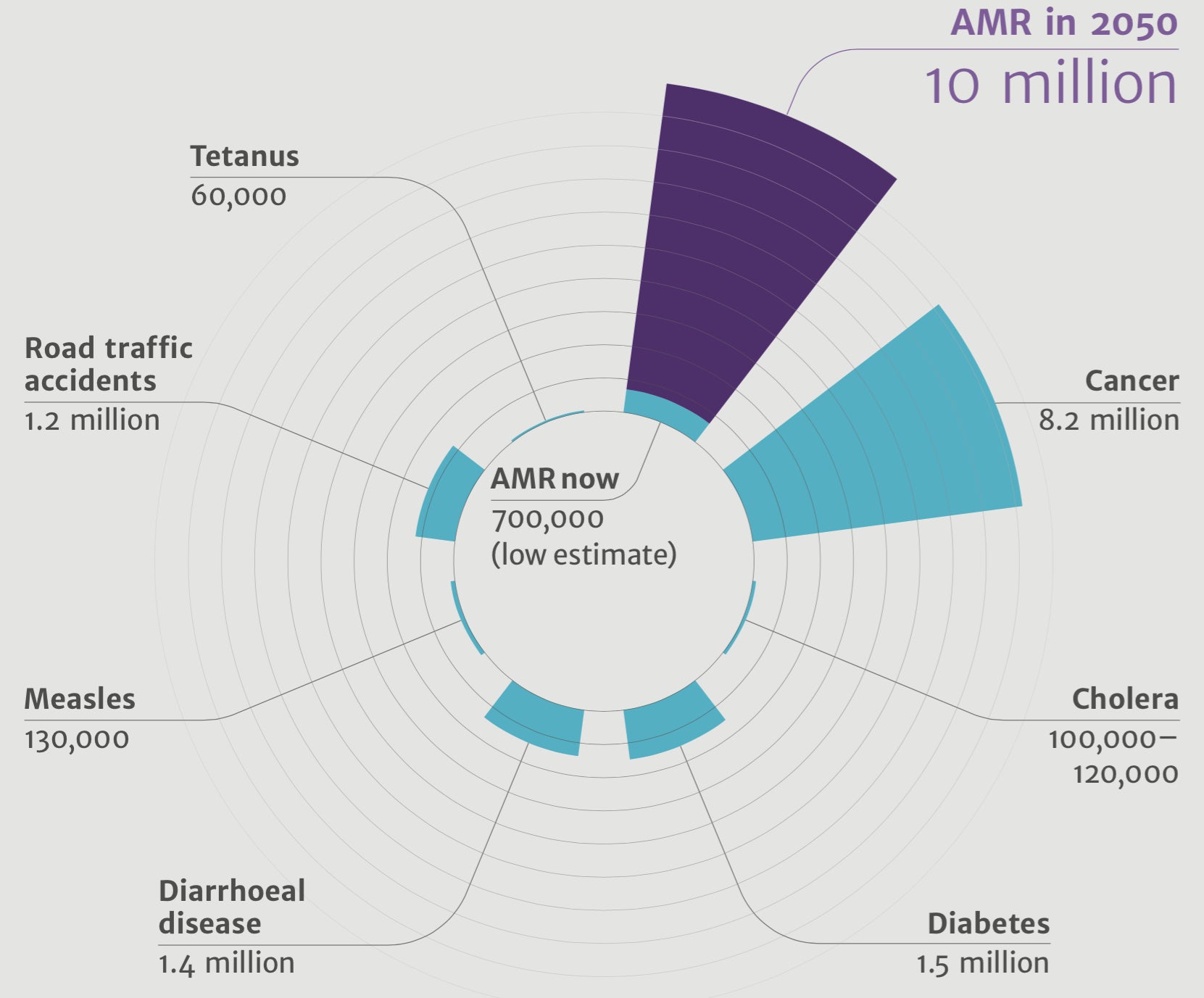
The Review on Antimicrobial Resistance estimates that globally, at least 700,000 people die every year due to drug resistance. That same report projects alarming costs if nothing is done: By 2050, 10 million people will die per year due to antimicrobial resistance. That’s more than the number of people who die from cancer, and it would come at a $100 trillion cost to the economy.
A world without effective antibiotics would mean a world in which procedures that we now consider serious, but routine — Caesarian sections, joint replacements, chemotherapy — would be highly risky or, in some cases, inadvisable due to the danger of infection.
There are many reasons behind the uptick in resistance, including the overuse of antibiotics in both humans and agriculture and the wily nature of bacteria themselves.
Bacteria live in a world in which they are constantly under attack, so to survive they have developed mechanisms to evade their attackers.
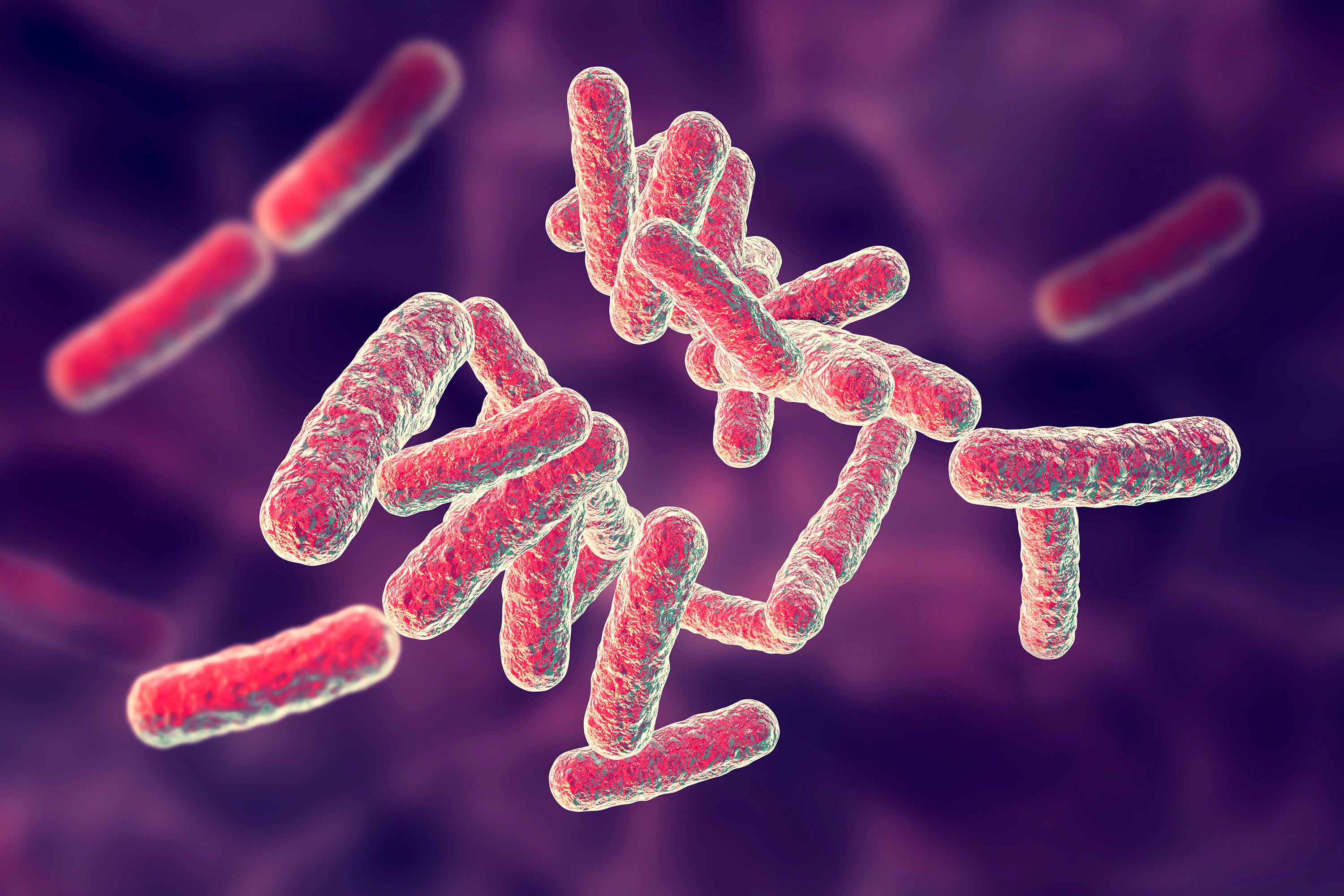
So-called gram negative bacteria — which include drug-resistant strains of pneumonia, gonorrhea, and E. coli — have “walls” around them, additional outer shells that protect them from drugs getting in. They also have pumps that pump the drugs out, so that people require higher and higher doses of antibiotics to kill them. They also mutate rapidly and, through a process known as “horizontal transfer,” are able to confer resistance on other, completely unrelated bacteria.
This, ultimately, is what can lead to superbugs, for which there is no cure.
“Sometimes, you’ll hear that something kills 99% of bacteria,” Patel said, “but then there’s always that 1% that somehow acquires resistance, either through some kind of transfer or just random mutations.”
Patel’s research, which she presented at the symposium, involves injecting antibodies into a patient, harnessing the immune system to fight off the infection.
“We’re trying a new approach where we can deliver this directly to people, so your body’s own cells start to produce these antibodies and will hopefully directly target the bacteria.”
The idea is a bit like CAR-T, the cutting-edge cancer therapy in which a patient’s T cells are extracted and modified to kill cancer cells, at which point they are injected back into the body and go to work.
In Patel’s approach, which is still in development, doctors would inject a DNA plasmid straight into a patient’s muscle. The plasmids are safe and non-infectious, and the process would be cheaper, since nothing needs to be modified or purified outside the body.
Researchers discussed other approaches, as well, including using a cocktail of antibiotics, similar to the way HIV is treated with a combination of different therapies. There are also bacteria-fighting viruses known as bacteriophages and vaccines to which bacteria show very little resistance.
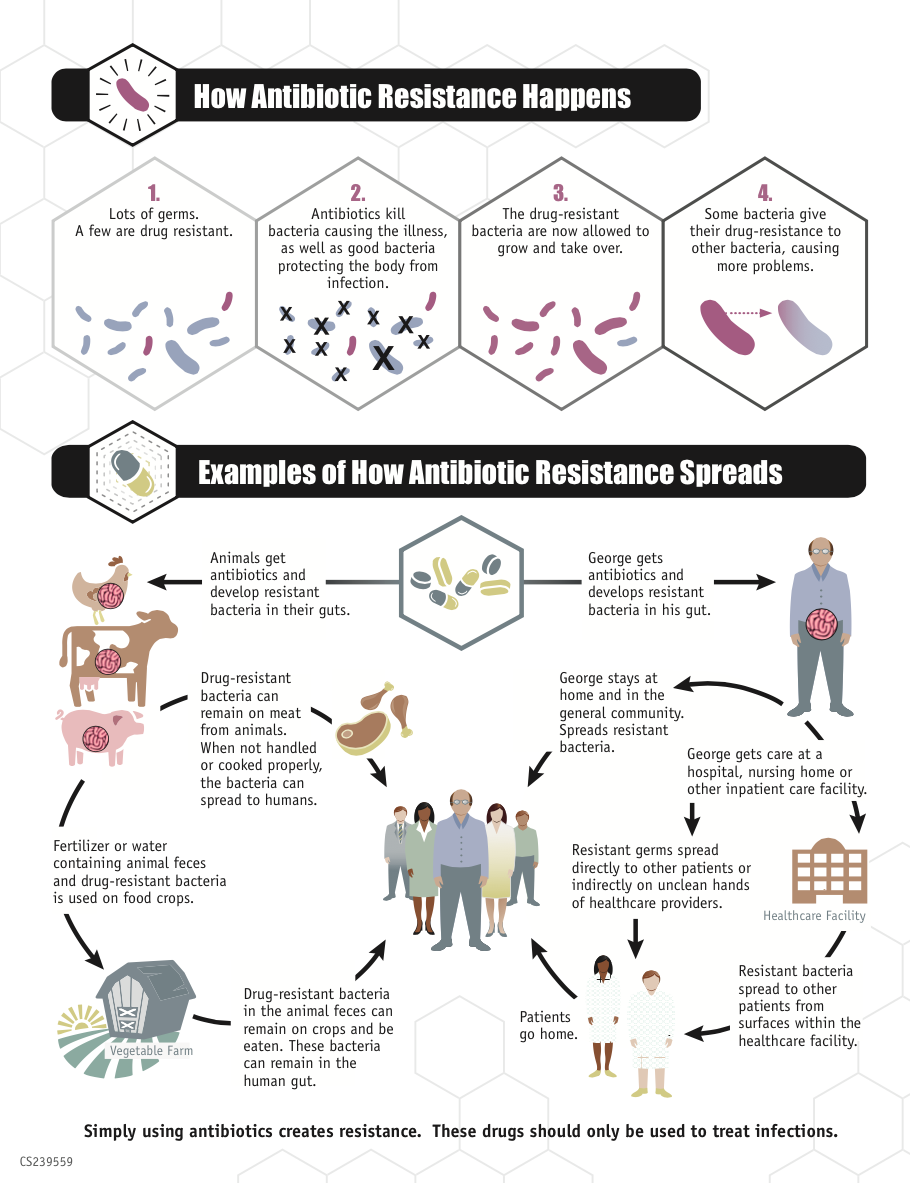
(Infographic courtesy of the Centers of Disease Control and Prevention)
‘A matter of life and death’
For Emily Kramer-Golinkoff, effective alternatives can’t come soon enough.
“For patients like me, it’s a matter of life and death.”

Kramer-Golinkoff has cystic fibrosis, which causes the mucus in her lungs to become thick and sticky like Silly Putty. Bacteria get stuck in the thickened mucus and cause infections, which require antibiotics.
“The best-case scenario is that you rotate your antibiotics,” said the 34-year old, who runs the cystic fibrosis nonprofit Emily’s Entourage. “But I’ve run out of options.”
She spends four hours a day doing various medical treatments, including oral and inhaled antibiotics, only one of which still works well for her. She worries alternatives to antibiotics won’t come fast enough.
“I can’t tell you how many friends I’ve lost in the last few years, the last few months, because of these issues,” she said. “Our options are running out, and they’re not being replenished. And I’m seeing people drop dead around me, and knowing that is my future as well unless something is done.”
Weiner said researchers around the world need to take this issue extremely seriously — not only for patients like Kramer-Golinkoff, who are especially at risk, but also for a population that is growing older and therefore, more susceptible to infection.
“The situation will continue to grow worse until we put in the resources and time and solve this problem,” he said.
“This is an important, line-in-the-sand time for us to refocus, reenergize, and deal with this important issue.”
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.





