Encouraging results from experimental Alzheimer’s drug
Results from an early trial give doctors hope that an effective treatment could be within reach.
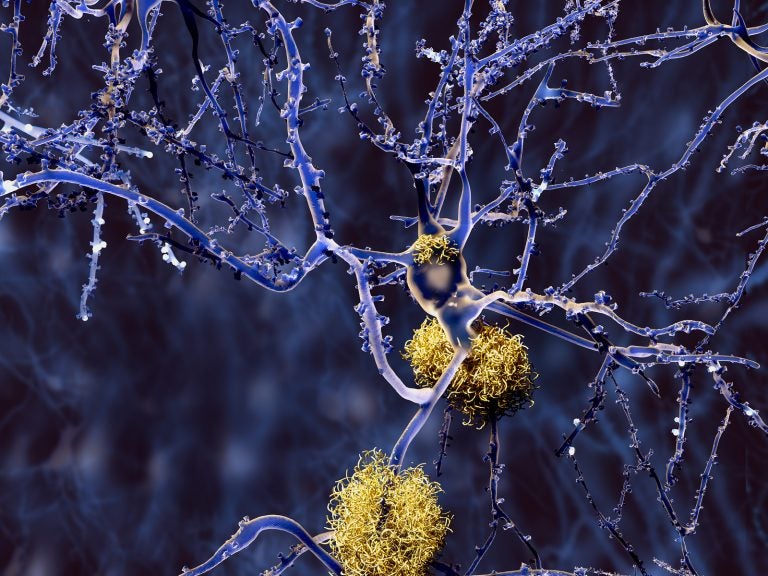
Amyloid plaques accumulate outside neurons. Amyloid plaques are characteristic features of Alzheimer's disease. (Bigstock/animaxx3d)
Finding a cure for Alzheimer’s disease has proved elusive. The drugs currently on the market only manage the symptoms of cognitive decline, and the most recent approval of one of those drugs was 15 years ago.
But new results from an experimental drug trial show a breakthrough treatment could be on the horizon.
The new drug, known as BAN2401, is being developed by the U.S. company Biogen and the Japanese company Eisai. Results from a phase 2 clinical trial show the drug removed some of the signature amyloid plaques that build up in the brains of people with Alzheimer’s and also slowed the progression of dementia.
That means it did something drugs currently on the market do not: It modified the disease, instead of just managing it.
“It’s a very promising result,” said neurologist David Wolk, who co-directs the Penn Memory Center. He attended the Alzheimer’s Association International Meeting in Chicago, where the results were released.
“We used to have to squint and look at things to feel like, ‘OK, maybe this could work.’ I think this is really giving us a real feeling that we’re making a lot of progress.”
Wolk and other Alzheimer’s experts, while encouraged by the results, also cautioned that more research is needed before the drug could be prescribed to patients. It was only a phase 2 trial, meaning it was smaller than the phase 3 trials typically required for FDA approval.
The slowed cognitive decline and reduction in amyloid plaques also only occurred in the group of trial participants receiving the highest dose of BAN2401, 10 milligrams per kilogram of a patient’s weight injected every two weeks; only 161 patients were in that group, which was one of six, including a placebo group. There were 856 patients in the trial overall.
Within the high-dose group, there was an underrepresentation of patients with the APOE4 genetic mutation, which is thought to be correlated with faster cognitive decline. Regulators had asked Biogen and Eisai not to include patients with the APOE4 mutation in the high dose group because in previous studies, drugs have caused side effects of brain swelling and brain bleeding in those patients.
But the placebo group did have patients with APOE4 mutations, which raises some questions.
“Is the medicine really helping mentally?” said Carol Lippa, director of the Cognitive Disorders and Comprehensive Alzheimer’s Disease Center at Jefferson Health. “Or are the people with genetic markers in the sugar pill group?”
Still, with 5.7 million Americans living with Alzheimer’s at a cost of $277 billion, according to the Alzheimer’s Association, Lippa says the study is exciting.
“I hope it does cross the finish line,” she said.
The patients in the study showed mild cognitive impairment, meaning they had mild symptoms and were in very early stages of the disease. That may be the key in future treatment of the disease. Earlier studies had focused on people with more advanced forms of the disease, at which point millions or billions of brain cells had already been lost.
“There’s a lot of reason to believe that trying to treat the disease before there’s a lot of damage and injury to the brain may be the most effective way to treat it,” Wolk said.
Biogen is also developing another Alzheimer’s drug called aducanumab, which is currently in two parallel phase 3 trials. Aducanumab is similar to BAN2401 in that it targets amyloid proteins before they clump together into plaques in the brain. The results from those trials likely won’t be available until 2020.
That may sound like a long time for someone recently diagnosed with the disease, but Wolk said there is hope that if either of these drugs are proved to work, there will still be options for recently diagnosed patients.
“Many people who are still in very early stages of the disease may be in very early stages of the disease over the next couple years,” Wolk said.
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.





