‘Right to Try’ may not meaningfully change access to drugs for dying patients
Yardley woman waits to see if new law allows her husband to get experimental medication for ALS.
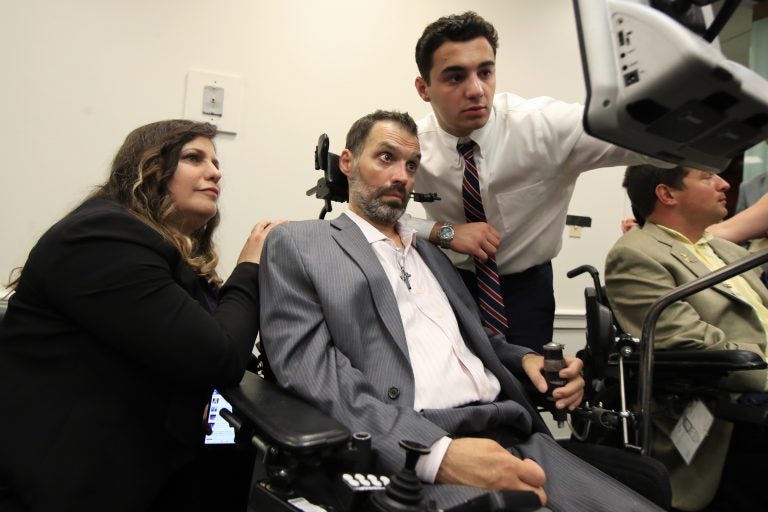
ALS patient Frank Mongiello communicates with his wife, Marilyn, and his son during a news conference following the passage of the "Right to Try" Act. The Yardley, Pennsylvania, family is hoping Frank Mongiello will be able to get experimental medication. (Manuel Balce Ceneta/AP Photo)
The “Right to Try” law, signed this week by President Donald Trump, will allow patients with terminal diseases to access drugs in the testing phase — once those patients have exhausted available approved treatments. What is unclear, however, is whether patients will actually have a better shot at using those drugs.
One Yardley, Pa., resident is hoping they will. Marilyn Mongiello has been pushing for the “right to try” since her husband, Frank, was diagnosed with ALS two and a half years ago. The fast-progressing disease has robbed him of his ability to walk, speak, and eat.
Once her husband’s disease was diagnosed — and she saw how quickly his health was deteriorating — Mongiello reached out to BrainStorm Cell Therapeutics to see if he could try NurOwn, an experimental ALS treatment in phase three of its clinical trial. The small company refused because it didn’t have a formal program that would allow patients to use a drug still in development.
Now, following the passage of the national law, the company plans to issue a formal policy on “right to try” in early June. Mongiello said she is eagerly awaiting the announcement.
“This may not be a cure for my husband, but at least I know we’ve exhausted every possibility,” she said. “It gives us hope.”
Patients already could apply to take such drugs under the FDA’s “compassionate use” policy; approximately 99 percent of requests made that way are approved. The new law signed by the president allows patients to do an end-run around the FDA, requesting the drug directly from the manufacturer. And it provides liability protection for drugmakers and doctors who offer the experimental drugs.
Even under the new law, drugmakers are not required to approve patients’ requests. It will also be easier for companies to charge higher prices for drugs that have only passed the first phase of a clinical trial, which shows they don’t have obvious safety problems.
That could be an incentive for drugmakers to offer drugs they otherwise might not. But Robert Field, professor of law and public health at Drexel University in Philadelphia, said it might not be worth it for drugmakers to divert the supply of an experimental drug for a small number of people.
“Manufacturers are not usually eager to provide medications in this way,” he said. “Their main interest is completing a clinical trial so that they can get the drug to market because that’s where the money is.”
It could also be tricky for manufacturers to know how to price the drugs. If they charge too much, they could be seen as heartless, since insurance likely won’t cover the cost; if they charge too little, if could be difficult to charge more when the drug reaches the market.
Mongiello said she does worry about the cost if and when her husband is able to try an experimental treatment.
“I hope the right to try won’t just be the right for the rich,” she said.
While the idea of increasing access to treatments is admirable, Field said, in many cases it gives patients false hope.
“There tends to be this aura of ‘new drugs must be better’ — that new means improved [like] the latest smartphone must have new features that the old one didn’t have,” he said. “And that’s not always the case for drugs.”
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.