Local biotech company to begin human testing for Ebola vaccine
Listen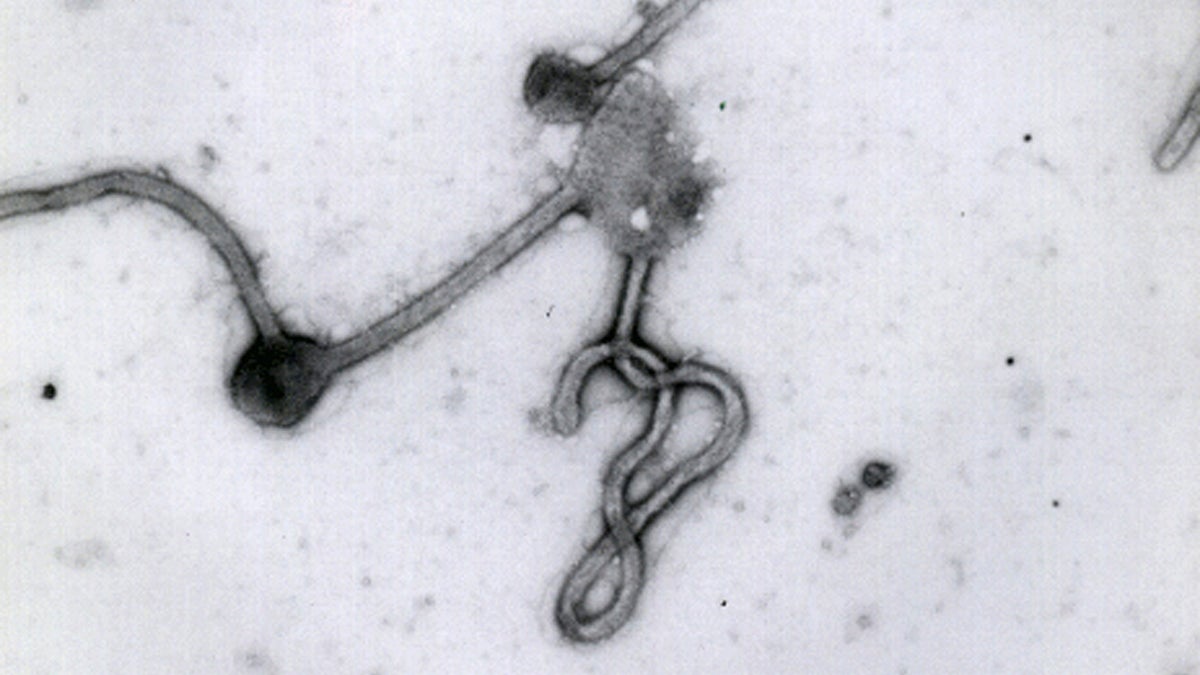
This undated photo made available by the Antwerp Institute of Tropical Medicine in Antwerp, Belgium, shows the Ebola virus viewed through an electron microscope (Antwerp Institute of Tropical Medicine/AP Photo)
Without any effective treatments for Ebola, health officials in West Africa are in desperate need of a vaccine to keep the virus from spreading. Stepping into that void, among others, is Inovio Pharmaceuticals of Plymouth Meeting, Pennsylvania.
President and CEO of Inovio, Joseph Kim, said beginning early next year their DNA-based vaccine will be given to 30 volunteers. The phase I human trial will test for safety and preliminary signs of efficacy based on analysis of blood samples for the appropriate immune responses.
The Inovio vaccine contains bits of Ebola virus DNA that correspond to its surface sugar coating, or glycoprotein. Once delivered into cells, those sequences can trigger the body to make protective antibodies and T cells that can go on the attack in case of infection. The technology was pioneered by David Weiner, a researcher at the University of Pennsylvania, who currently serves on Inovio’s scientific advisory board and also helped co-found a precursor to the company.
“What makes us different is our DNA-based vaccine approach that allows us to engineer a cross-protective immune response not just against a single strain,” said Kim, “but provide a greater, bigger umbrella-coverage.”
In earlier animal testing, the vaccine protected 100 percent of guinea pigs and mice from death after exposure to the virus.
While promising, Bruce Lee of the International Vaccine Access Center at Johns Hopkins University said that demonstrating a vaccine is safe and effective is only part of the task at hand.
“A big part is getting the vaccine out from the manufacturers to the country, and then from the central part of the country to all the outlying regions,” he said. “And once you get the vaccine out to all the different places, getting people to accept the vaccine is another big challenge.”
The logistical concerns are likely to be both easier and harder with a DNA vaccine. Mass production and transit should be easier, since DNA vaccines are quite stable. But administering them requires a special machine that delivers an electrical zap as a means for DNA to enter cells.
GlaxoSmithKline has already begun its own phase I trial with a more traditional vaccine. At least several other groups are following suit.
Medical director of Doctors Without Borders’ Access Campaign, Jennifer Cohn, said the key to making any Ebola vaccine useful to the current outbreak is ensuring the supply.
“What we don’t want to see is that several batches of vaccine are produced simply for the trial and then we are left in this valley where there is no product left,” she said.
Above all, Cohn said, regulators and manufacturers cannot delay.
“We need to push these vaccine candidates through as quickly as possible, because we don’t know when this outbreak will end, and a vaccine is incredibly important in case this outbreak does continue,” she said.
According to the World Health Organization, Ebola has killed more than 3,000 West Africans in the current outbreak — a number health officials agree likely underestimates the true death toll.
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.

