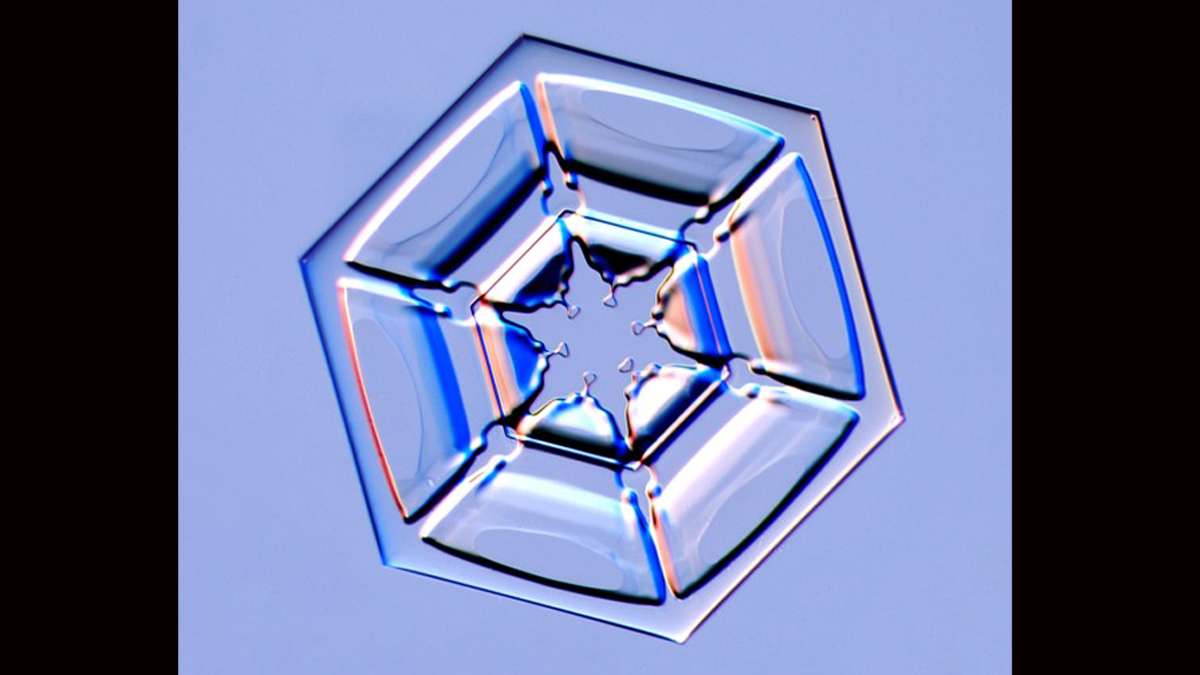
Why are no two snowflakes the same?
ListenAn end-of-winter reminder on the science and wonder of snowflakes.
Dirty snowdrifts and hard-packed piles can make it hard to appreciate snow at this time of year. In the extreme, snow’s a drag, but it’s easy to admire the pristine beauty of a single snowflake.
Here’s how they grow: Snowflakes start as water vapor in the clouds.
When the temperature is supercool—well below the 32-degree freezing point—vapor morphs directly into a solid snow crystal—usually after bumping into some microscopic particle or debris.
Soon, other water vapor condenses on the ice and the crystal grows bigger.
“Water molecules just get jostled around in the air until they find their way to the snowflake,” said Kenneth Libbrecht, a physicist at the California Institute of Technology. “Some of them will hit the crystal and some of them won’t, and that process is called diffusion, and we can model that mathematically.”
A snow crystal is a single piece of ice but the terms “snow crystal” and “snowflake” are often used interchangeably. For scientists, snowflake often indicates several snow crystals stuck together.
“Although we imagine them to be two dimensional objects, they are actually not. They extend in all directions—they have some thickness,” said Janko Gravner, a mathematician at the University of California, Davis.
Libbrecht says, at first, the crystal is squat and compact. It’s nothing like the lacy hexagonal shapes you’d see on a Christmas card. But, because of the way H20 molecules bond together snow crystals do have six-sides—right from the start.
“This is partly why the snowflake forms branches,” Libbrecht said.
As the snowflake grows, the outward tips of the hexagon stick out a little farther and are exposed to more fresh sources of water molecules.
“The water vapor will get to the tips more quickly than it gets to other parts of the crystals,” Libbrecht said.
That explains just some of the process. Libbrecht says there’s still lots of surface physics to figure out.
“It’s not that one has any practical applications of this,” he said. “You just want to see if you can understand what’s happening in nature.”
Constantly changing temperatures, pressures and humidity conditions give a growing snow crystal its shape as it moves through a cloud. It could become a familiar lacy star—scientists call that a stellar dendrite—but a snowflake can just as easily grow to look like a six-sided pencil.
When snowflakes grow as big as a 1/10th of millimeter or more, the water ice falls from the clouds.
Certain snowstorms produce just one crystal pattern.
“Some days you’ll go out and see nothing but canonical, star-shaped flake-like crystals,” Libbrecht said. “Sometimes you go outside and see mostly needles falling—depends on the temperature.”
Snowflake hunting
Libbrecht is a professor at Caltech—as well as a celebrated photographer and snowflake hunter.
All of us can study snow crystals using an inexpensive magnifying glass, but Libbrecht warns that the flakes lose their shape soon after they hit the ground.
“You got to get ’em when they are fresh and just falling. You want the temperature around 5 degrees Fahrenheit to get really nice crystals,” he said.
Northern Ontario, Northern Japan and Alaska are all good snowflake hunting grounds.
“New York, that’s too warm, Pennsylvania, too warm. Even the mountains most of the time are too warm,” he said.
To catch snowflakes in the wild, and create his beautiful photographs, Libbrecht custom-built a microscope and camera—all wrapped in insulation to protect the equipment from the cold.
“I will let snowflakes fall onto a piece of cardboard, and I’ll watch and sort of look for nice looking specimens, and if I see one I’ll pick it up using a little paintbrush and drop it onto a glass slide,” he said.
Mimicking nature in the lab
There’s a cold chamber at Caltech where Libbrecht creates designer snowflakes under controlled conditions.
He knows what conditions to mix together to get the snowflake shapes he wants but because the science is still somewhat a mystery at the molecule level—it’s not always clear what has happened.
It’s kind of like following a recipe for bread: you don’t need to know the chemistry of yeast to get good results. “You just follow the rules,” Libbrecht said.
At the University of California, Davis, mathematician Janko Gravner creates virtual snowflakes on his computer. He models the tiny temperature shifts that snowflakes encounter as they grow, and says if we could just read their patterns, we’d have a small history of where a snowflake has traveled.
“Nobody can hang in the cloud with a microscope and see how the snowflakes form,” he said. “They cannot just appear at random because the snowflakes are so symmetric.”
An enduring question
So, is it true that snowflakes are alike?
Scientists like to hedge their bet when they answer that question.
Gravner says—technically—two crystals could grow to be identical, but it’s extremely unlikely.
Consider again, all the different atmospheric conditions in a cloud. Even two snowflakes forming right next to each other experience the trip differently.
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.