What’s holding up coronavirus testing? Lack of necessary chemicals and lab equipment
Researchers say the U.S. needs to test at least 500,000 people a day. But labs and health systems have to share what’s required to perform the tests.
Listen 1:33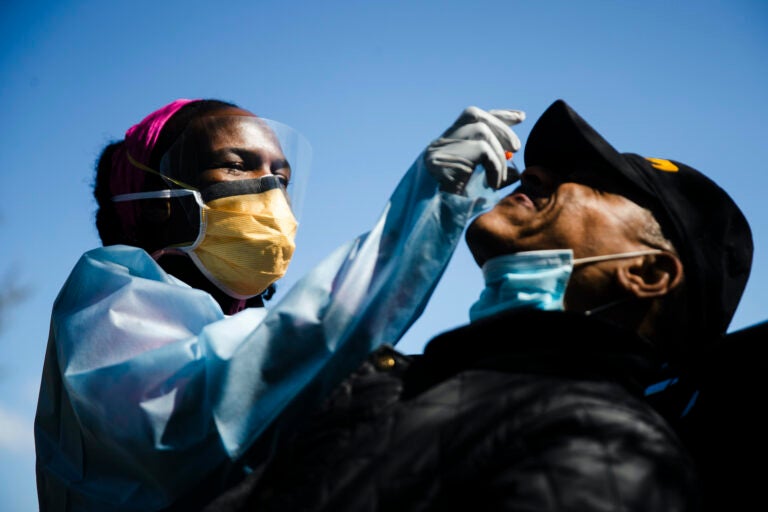
Dr. Ala Stanford administers a COVID-19 swab test on Wade Jeffries in the parking lot of Pinn Memorial Baptist Church in Philadelphia, Wednesday, April 22, 2020. (AP Photo/Matt Rourke)
The United States needs to do far more coronavirus testing, public health experts say, but the problem isn’t just related to the number of machines required to run the tests. There’s also a shortage of the supplies, such as chemicals and plastic lab equipment, that are needed to run those machines.
Last weekend, Vice President Mike Pence said the country is doing about 150,000 tests a day. Researchers at Harvard University estimate that the U.S. needs to test at least 500,000 people a day, if not more.
Bruce Meyer, president of Jefferson Health in Philadelphia, said the shortage of chemicals is what’s holding the health system back from doing more tests. Jefferson has 14 hospitals across Pennsylvania and South Jersey, including Abington Hospital, Jefferson Cherry Hill Hospital, and Thomas Jefferson University Hospital.
Meyer said the test chemicals come in early in the morning every day, but the health system finds out only a day or two ahead how much they can get. He said the Centers for Disease Control and Prevention advise companies where the disease hot spots are, and thus which places need tests more than others. Once Jefferson Health staff members get their daily supply of testing chemicals, they look at how many patients need tests and decide who gets one. They typically use up all the testing chemicals they receive.
“Supplies are really literally a day-to-day conversation with the commercial labs and the CDC,” Meyer said. “We’re still sending a lot of tests out to the commercial labs that we would rather do locally, if nothing else because of turnaround time.”
A CDC press officer said Wednesday that the agency distributes testing supplies to public health labs in a centralized way through the International Reagent Resource, to monitor access and control for quality. In the current pandemic, the supplies include swabs, test kits and test chemicals. The International Reagent Resource works with the Federal Emergency Management Agency and uses an algorithm to send testing supplies to labs.
These test chemicals are useful only to labs doing research on viruses, but all of a sudden every state wants them at the same time. That’s both surprising and frustrating to scientists, said Kristin Omberg, a chemist and lab manager at a nonprofit research institution studying the new coronavirus.
“Every major supplier that I’m talking to is telling me, ‘I don’t know when I’ll get these in, but I’ll ship them to you when I get them in, based on where you are on my wait list,’” she said. “It’s like toilet paper; it’s maddening. These things are never in short supply.”
Omberg said every place that wants to test for the coronavirus has to choose from a list of parts the Food and Drug Administration provides, as part of the emergency use authorization for each approved test. If a lab does not use those parts or those chemicals, they cannot use the test results to make clinical decisions.
To test someone for the virus, a health care worker uses a swab that goes all the way to the back of the nose to collect a sample. That sample has to go into a mix of chemicals and nutrients called viral transport media to keep the virus, if it’s there, intact until it gets to a lab. Then the lab puts the sample in another mix of chemicals called a lysis buffer, which makes the virus relatively harmless but keeps the genetic material intact, so people in the lab can test for the genetic material in the virus without worrying too much about becoming infected themselves.
All those things — the various chemicals, the swabs, even the plastic pipette tips used to transfer bits of virus from one place to another – can be hard to get now, Omberg said.
Different testing procedures approved by the FDA, called assays, require different combinations of parts, but the parts are specific to each approved procedure and are not interchangeable. Labs have resorted to trading parts back and forth, Omberg said.
“Different labs will contact each other and ask, ‘Do you have any of this? Could you send me some of this, if you have some that you’re not using? … If you’re not running this particular assay, can we have all of your pieces of that particular assay, and we’ll barter you for some of our buffer for the kit that you need?’”
Thermo Fisher Scientific, one of the companies that makes authorized testing supplies and test kits, has not had any trouble meeting demand for its various products, according to Ron O’Brien, senior director of public relations. He said the company is in touch with states across the country, including Pennsylvania, Delaware and New Jersey, to find labs with unused lab equipment that the state could loan to run COVID-19 tests. That happened earlier this month with a piece of equipment from the Shedd Aquarium in Chicago.
In the meantime, Jefferson Health’s Meyer said the various health care providers in the region work with one another on testing. For example, Temple Health and Jefferson have provided testing supplies for each other, according to a Temple representative.
“There is not going to be a significant explosion of testing capability from what we have today,” Meyer said.
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.




![CoronavirusPandemic_1024x512[1]](https://whyy.org/wp-content/uploads/2020/03/CoronavirusPandemic_1024x5121-300x150.jpg)