Penn scientists reprogram gut cells to produce insulin
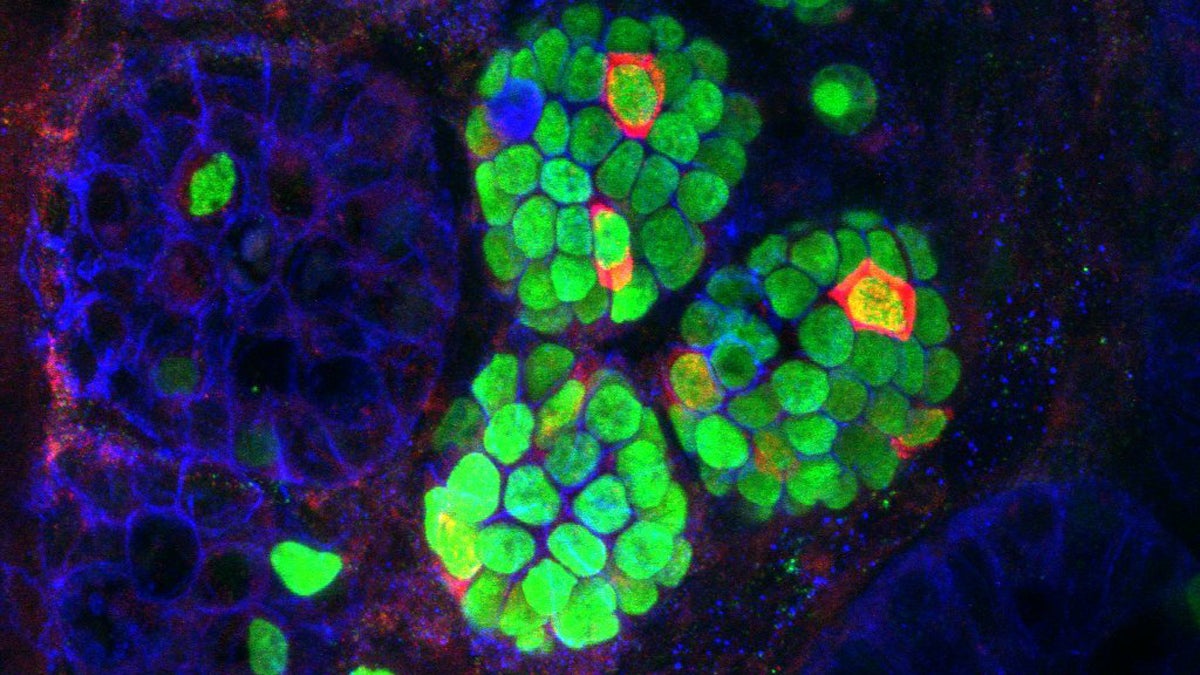
Insulin-expressing cells (red) arising within the intestinal crypts (green) of a mouse that received three beta-cell 'reprogramming factors (Image courtesy of UPenn)
Researchers are getting closer to figuring out how to transform gut cells into insulin powerhouses.
The only cell in the body that makes insulin — the critical hormone that regulates blood sugar — is the beta cell in the pancreas. That’s a problem for diabetics, who either have dysfunctional beta cells or immune systems that kill off too many of them.
But researchers at the University of Pennsylvania are getting closer to figuring out how to transform highly abundant gut cells into insulin powerhouses.
In 2008, stem cell scientists in Boston found they could reprogram one type of pancreatic cell into a beta cell by forcing the expression of three specific genes. To see if any other types of cells could be reprogrammed, Ben Stanger, a physician-scientist at Penn, began by putting the three factors into a bunch of different tissues in mice.
“The new insulin-producing cells that we found came from a portion of the intestine right next to the pancreas,” said Stanger. “And that indicates that those intestinal cells have some memory going back to the time that they were embryonic, of what they might have been able to become. So it didn’t take quite so much to be able to push those cells to become pancreatic, and to become beta cells.”
When Stanger tested the reprogrammed cells, he found they spit out insulin only in response to sugar, just like real beta cells. They aren’t completely identical, but they were able to lower blood sugar in diabetic mice. Preliminary testing suggests the trio of factors also refashions human intestinal cells into quasi-beta cells. The results were published in the journal Cell Reports.
“Our hope would be that we can then take patient-derived intestinal cells, reprogram them into cells that look and function close enough to beta cells that they could be used in the patient for treatment of diabetes,” said Stanger.
That would be akin to an islet transplantation that is already sometimes done for autoimmune diabetics, but is limited because those cells must come from donors.
Right now, Stanger said, they’re at least five years away from moving toward clinical use. He first needs to maximize the amount of insulin the intestinal cells produce, and work more with reprogrammed human cells to see if they perform as well as in mice.
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.

