New Jersey is ‘aggressively pushing back’ against Texas abortion pill order, Murphy says
Though the Garden State codified abortion protections, Gov. Phil Murphy said he’s concerned about what a potential ruling could mean for existing protections.
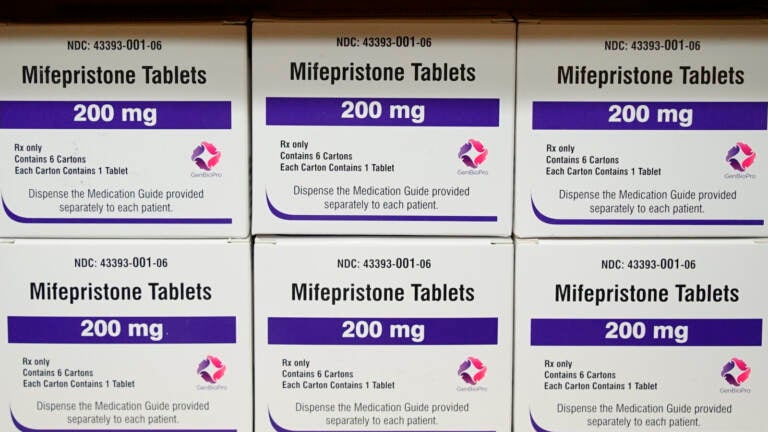
File photo: Boxes of the drug mifepristone sit on a shelf at the West Alabama Women's Center in Tuscaloosa, Ala., on March 16, 2022. Attorney generals in 20 conservative-led states warned CVS and Walgreens on Wednesday, Feb. 1, 2023, that they could face legal consequences if they sell abortion pills by mail in those states. (AP Photo/Allen G. Breed, File)
New Jersey has become a refuge for people seeking reproductive health services banned in other states like Texas and Alabama. Still, a federal court case could decide whether doctors can continue prescribing a widely used abortion pill, nationwide.
Though the Garden State codified abortion protections, Gov. Phil Murphy said he’s concerned about what a potential ruling could mean for existing protections.
“We are aggressively pushing back on the decision that was handed down in Texas,” Murphy said.
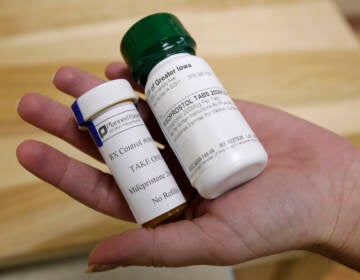
Speaking on WHYY’s monthly call-in show “Ask Governor Murphy” on Tuesday, Murphy said his administration supports the U.S. Justice Department appeal this week of a Texas judge’s ruling that revokes the FDA approval of mifepristone, one of two abortion medications used in the country.
A federal appeals court ruled Thursday that the medication can still be used for now, but said it could not be dispensed by mail. It also reduces the time in which a person can use the drug from up to the tenth week of pregnancy, to the seventh.
Murphy said it could throw New Jersey’s laws in flux, including laws he signed last summer protecting doctors who offer telehealth services to people seeking abortions from out of state.
The Democrat said New Jersey has filed an amicus brief alongside the Biden Administration.
“We’re trying to consider what other states are also considering: whether or not we want to go out there and aggressively stockpile one or both of these pills,” Murphy said.
The judge in Texas did not halt the approval of another common abortion pill, called misoprostol. Several pharmaceutical company executives signed a letter cautioning that the ruling could impact other medications, according to the Associated Press.
The Alliance Defending Freedom, the group behind the Mississippi case that overturned Roe v. Wade, has filed the lawsuit. The group’s position is that the FDA’s initial approval of mifepristone was flawed due to insufficient safety risk assessment, the Associated Press reported.
Traditionally, courts have deferred to the FDA on drug safety and efficacy matters. However, the agency’s authority is being tested in a post-Roe legal landscape where several states have banned or limited access to abortions and enacted laws targeting abortion medications.

Get daily updates from WHYY News!
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.