A Nobel Prize for noble gases
William Ramsay in 1904 (Munn & Co./Appleton's Magazine)
Today the periodic table is a familiar sight in science classrooms. It takes the 118 elements that compose everything in the known universe and arranges them so that substances in any given column share similar chemical properties.
Some of these elements, like gold, have been known about since antiquity, but those in the rightmost column were only added to the table in the late 19th century, thanks to chemist William Ramsay.
Ramsay was born in Glasgow on October 2, 1852. He grew up in a scientifically inclined family — his grandfather was a dye manufacturer and his uncle was a well regarded geologist — but only became interested in the subject after he broke his leg playing football. To pass the time during his recovery, Ramsay read chemistry textbooks and eventually assembled a small laboratory in his bedroom, where he tried to make his own fireworks. These experiments would later inspire him to pursue a doctorate in chemistry. He secured teaching positions in Glasgow and Bristol before becoming chair of inorganic chemistry at University College, London in 1887.
By the time he reached London, Ramsay had acquired a reputation as an excellent experimentalist and had published several papers on the chemistry of nitrogen. This expertise proved valuable in April 1894, when he attended a lecture at the Royal Society by Lord Rayleigh, a physicist who had noticed a strange discrepancy while measuring nitrogen’s density. It appeared that nitrogen isolated from the air was slightly heavier than nitrogen produced through chemical reactions, such as the decomposition of ammonia. Intrigued, Ramsay approached Rayleigh after his talk to propose a collaboration.
Together the two men determined the underlying reason for Rayleigh’s confusion. Ramsay subjected samples of atmospheric air to a barrage of reactions to eliminate any trace of oxygen or nitrogen. By early August 1894, he had isolated the anomaly — a previously unknown gas in the atmosphere that refused to react with any other element. Because of this sluggish behavior, Rayleigh and Ramsay called the new gas “argon,” a name derived from the Greek for “inactive.”
Reaction to Ramsay and Rayleigh’s findings were mixed. Dmitri Mendeleev, the Russian chemist credited with creating the periodic table, initially denied that argon was an element, suggesting it could be a previously unknown form of triatomic nitrogen (N3). He changed his mind after Ramsay discovered additional gases that shared argon’s chemically inert nature.
In 1895, Ramsay successfully isolated helium and during the summer of 1898, he identified three more “noble” gases — a description reflecting their apparent unwillingness to participate in chemical reactions: neon, krypton, and xenon.
Over a few short years, William Ramsay had added an entirely new column to the periodic table. The noble gases he revealed to the world soon found a home in applications ranging from neon lights to liquid helium cooling systems. In recognition of these accomplishments Ramsay became the first British scientist to earn a Nobel Prize in chemistry in 1904, and his friend Lord Rayleigh won the physics prize that same year.
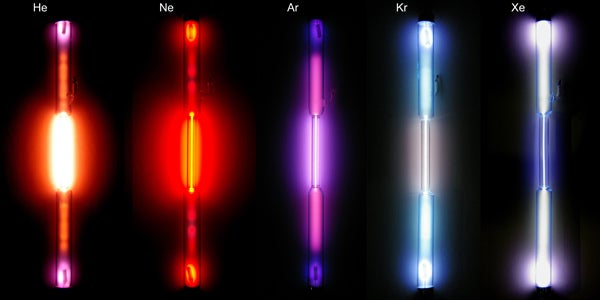
Noble gas discharge tubes; from left to right: helium, neon, argon, krypton, xenon. (Alchemist-hp/wikimedia commons)
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.



