Hospitals promote heart procedure without disclosing risks, Penn study finds
Listen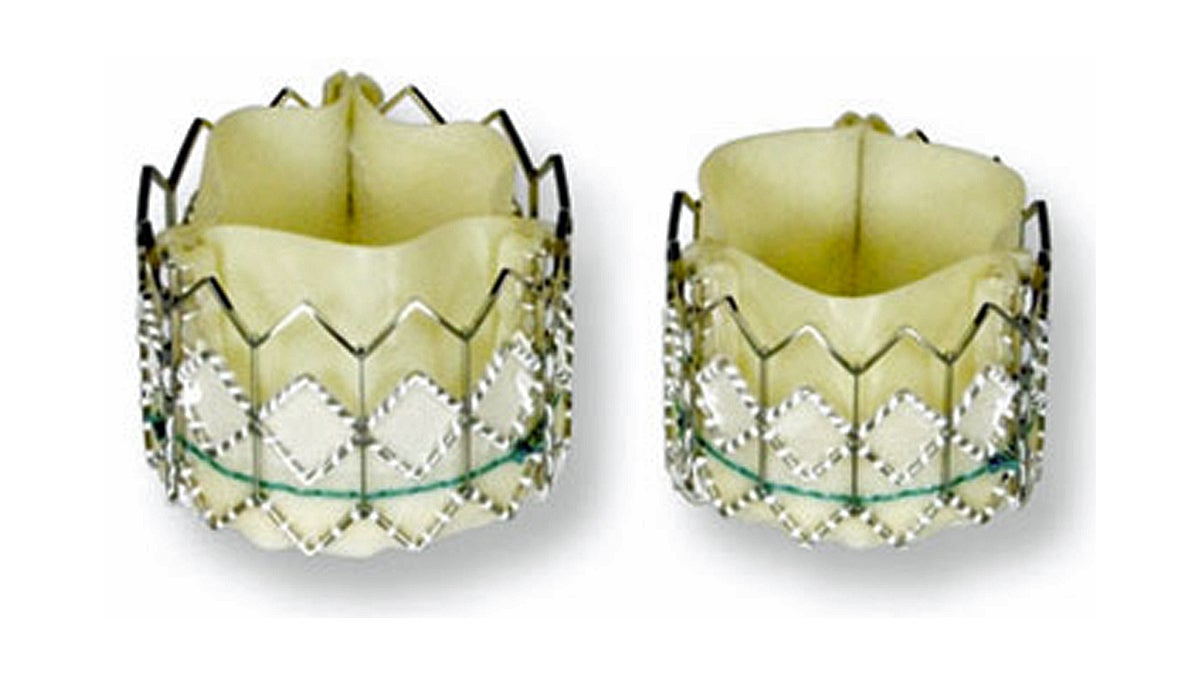
Edwards SAPIEN Transcatheter Heart Valves, Tri-leaflet bovine pericardial tissue treated with TermaFix Process (left) and a balloon expandable stainless steel stent for sutureless implantation. (Image via FDA)
Just three years ago, doctors pioneered a new technique to replace an aortic valve by threading in a replacement — no open heart surgery necessary.
The transcatheter aortic valve replacement, as it’s known, is a cutting-edge procedure, allowing many patients who would have been unable to tolerate a more typical surgery to have their hearts repaired. But it still comes with risks.
According to a new study from the University of Pennsylvania, hospitals have been advertising the procedure without mentioning those dangers.
Penn anesthesiologist Mark Neuman and his team surveyed the websites of more than 250 hospitals in the U.S. that offer the procedure. Nearly every one described at least one benefit, such as a faster recovery time or minimal invasion. But only 26 percent of the hospitals mentioned any risks.
“Some of these risks, including potentially stroke or vascular complications, can be quite serious,” said Neuman.
Hospitals were also far more likely to quantify the benefits of the procedure than the risks.
Even though anyone undergoing the surgery would still consult with a doctor, Neuman said he found the imbalance of information worrisome for patients.
“The sort of messaging that they see on the web or in the media can potentially set the tone for these decisions or influence their decision in potentially important ways,” said Neuman.
Professor of health care strategy and marketing at St. Joseph’s University, Bill Trombetta, agreed that hospitals should be more careful with their descriptions.
“It’s risky business to run any kind of health care ad because to the extent that a consumer relies on things, maybe you’ve painted too rosy of a picture of the device,” he said. “Then I think you could then be in some difficulty.”
Legally, hospitals are not bound by the same marketing rules that prescription drugs are, which are required by the Food and Drug Administration to be presented in a fair and balanced way, and therefore often come with a comically long list of potential side effects. Rather, medical procedure promotion falls under the purview of the Federal Trade Commission, which also regulates ads for consumer products such as cereal.
Neuman said his work, which was published this week in the journal JAMA Internal Medicine, shows why patients should seek out multiple sources when researching medical procedures.
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.

