I’m pregnant. Is the COVID-19 vaccine safe for me and my baby?
What is the risk, if any, while someone is expecting? WHYY’s Health Desk Help Desk looked into what the research is showing.
Listen 2:27
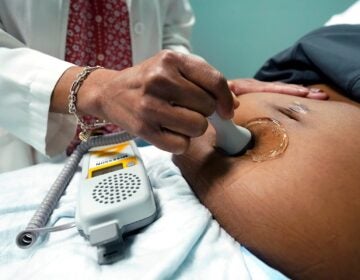
A medical professional holds a stethoscope on a pregnant person's belly. (VadimGuzhva/Bigstock)
Ask us about COVID-19: What questions do you have about the coronavirus and vaccines?
This is one of a series of articles in which reporters from WHYY’s Health Desk Help Desk answer questions about vaccines and COVID-19 submitted by you, our audience.
The Centers for Disease Control and Prevention recommended in April that individuals who are pregnant or lactating get the COVID-19 vaccine, noting that the benefits outweigh the risks. Here’s what health experts know so far about the coronavirus and the vaccine within these groups.
What are the risks of COVID-19 during pregnancy?
In general, those who get COVID-19 while pregnant are at a slightly increased risk for more severe illness. That means an increased chance of being admitted to the intensive care unit, an increased need for breathing assistance, and an increased risk of death compared with nonpregnant patients.
Pregnant patients with comorbidities, and older patients, have an elevated risk of adverse medical events. The CDC found that pregnant women between the ages of 35 and 44 were close to four times as likely to require invasive ventilation, and twice as likely to die from COVID-19 than were non-pregnant women of the same age.
The CDC reports that the increased risk during pregnancy might be related to physiologic changes such as increased heart rate and oxygen consumption, decreased lung capacity, a shift away from cell-mediated immunity, and increased risk for blood clots and associated complications.
Pregnant people also have a higher risk for severe illness from other infections, such as the flu. “And we think that that partly is related to some of the physiology and physiologic changes in your different organ systems in pregnancy,” said Dr. Sindhu Srinivas, director of obstetrical services at the Hospital of the University of Pennsylvania.
“In COVID-19, I think there’s also probably a combination where some of it might have to do with some of the limitations and information that we’re obtaining, meaning that when pregnant women get sick with different viruses or different illnesses, we do tend to admit them to the hospital a little bit more frequently, potentially send them to the ICU a little bit more frequently, because of an increased level of care. So I think it’s a combination of being cautious about making sure we’re providing the highest level of care in a population that we know is at higher risk for severe disease. And the severity of illness that we see with COVID, that it is a little higher in pregnant women, is similar to what I’ve seen in other viral illnesses like the flu.”
Is the vaccine safe for people who are pregnant?
Pregnant people were not included in the original COVID-19 vaccine trials. But in April, the CDC began recommending the vaccine after preliminary data published in the New England Journal of Medicine showed that both the Pfizer and Moderna shots were safe for pregnant women and their babies. The study followed 35,691 women.
CDC officials said last month that they are expecting data from trials testing the vaccines on pregnant women this summer. While that data is being collected, health experts say the benefits outweigh the risks.
“So far, what we see is that there’s been many women while pregnant who have received the vaccine. And while it’s unfortunate patients who were pregnant were not included in the original vaccine trials, so our data is limited in terms of their inclusion in the trials, vaccinations have started and many women who are pregnant have received the vaccination. The safety concerns theoretically were small to begin with, and since the vaccinations have happened, there has been no signal at all of any safety concerns about pregnancy and people receiving the vaccine,” Srinivas said.
Pennsylvania’s acting state physician general, Dr. Denise Johnson, said any of the three currently approved vaccines are appropriate for pregnant women.
Are there any rare vaccine risks?
Last month, Brazil’s government suspended use of the AstraZeneca vaccine for pregnant women, after an expectant mother died from a stroke.
In general, pregnant patients also have a slightly higher risk of getting blood clots compared to non-pregnant patients.
In the United States, the federal government briefly paused administering the Johnson & Johnson vaccine after some non-pregnant women had serious but rare blood clots. The CDC recommended resuming the use of the vaccine, but said women under 50 should be aware of the rare risk.
Srinivas said that it’s important to remember that those incidents were extremely rare, and that the benefits of the vaccine still outweigh the risks.
“When you vaccinate millions of people, there are rare risks you might see … But in general, we feel like, even with these very rare reports, that the benefit of vaccination outweighs any of these rare risks,” she said.
What about those who are lactating?
The vaccine doesn’t pose any risks to lactating people or their infants. And early data shows that vaccinated mothers can transfer their antibodies to their babies while breastfeeding.
“Vaccination is absolutely recommended for lactating people,” Srinivas said. “There’s so much vaccine safety data at this point, and there’s actually a benefit to the individual, benefit for the baby, as well as the population benefit as well. And so I think lactating patients in general should be getting vaccinated.”
Is the vaccine effective during pregnancy and postpartum?
Available data shows no concern about efficacy. Srinivas said there’s no reason to believe that the vaccine would be less effective in people who are pregnant or lactating.

Get daily updates from WHYY News!
WHYY is your source for fact-based, in-depth journalism and information. As a nonprofit organization, we rely on financial support from readers like you. Please give today.



![CoronavirusPandemic_1024x512[1]](https://whyy.org/wp-content/uploads/2020/03/CoronavirusPandemic_1024x5121-300x150.jpg)