Will gathering vast troves of information really lead to better health?
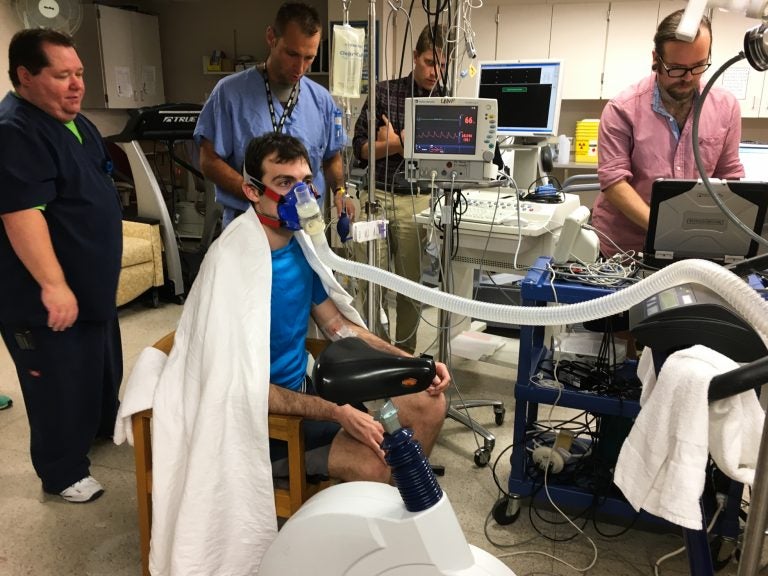
Volunteer Greg Ruegsegger is outfitted with monitors, a catheter threaded into a vein and a mask to capture his breath in an experiment run by Joyner to measure human performance. (Richard Harris/NPR)
The Mayo Clinic is building its future around high-tech approaches to research known as “precision medicine.” This involves gathering huge amounts of information from genetic tests, medical records and other data sources to ferret out unexpected ideas to advance health. But one longtime scientist at the Mayo Clinic isn’t playing along.
Dr. Michael Joyner is a skeptical voice in a sea of eager advocates. Joyner’s lab studies exercise. It is, fittingly enough, in a hospital building founded in the 1880s. While Mayo has built all sorts of new labs at its sprawling campus in Rochester, Minn., Joyner can conduct his work without glitzy DNA sequencers and other high-tech tools of precision medicine.
And it’s not simply that he’s an old-school devotee. He believes that the solution to our most pressing health problems lies in thinking about whole human beings, not breaking everything down to DNA sequences.
“The enthusiasm for this [precision medicine] is occurring in a country where life expectancy is actually falling,” he says as he walks through the old linoleum-tiled halls of St. Mary’s hospital.
That fact alone leads Joyner to ask whether the money being poured into high-tech medical research is really solving the nation’s stark health problems, like obesity, heart disease, high blood pressure, diabetes, Alzheimer’s disease and cancer.
Joyner says there are certainly appropriate places to use this technology, and he doesn’t dispute the individual stories of success his colleagues tout. But he believes the best way to address the health concerns facing the nation is by studying — and treating — whole people, not by breaking the problems down to billions of genetic bits and pieces.
He practices what he preaches in both his personal life — his thorough exercise routine includes commuting by bicycle — and his physiology lab.
On the day I visited at the end of August, volunteer Greg Ruegsegger was outfitted with monitors, a catheter threaded into a vein and a mask to capture his breath. He would exercise to the point of exhaustion while scientists studied him. This is far more informative than any genetic test, Joyner says.
“People have looked at 3,000 elite endurance athletes — these are people who compete in the Tour de France and win Olympic medals in cross-country skiing and distance running — and [scientists] have been unable to find any genetic marker for superior performance.”
And when it comes to the health of ordinary people, Joyner talks about real-world studies, which show that walking or biking to work has four- or fivefold more influence on a person’s body mass index than that person’s genetic profile.
Joyner is focused on how the body’s systems work together during exercise, but the same interconnectedness applies to many diseases. That’s why cancer drugs that target one biological pathway generally don’t last long. Tumor cells simply find workarounds, exploiting the redundancies deeply embedded in biology. And that’s why Joyner has so little faith in science that keeps trying to focus down on smaller and smaller details.
“One of the things we have to ask ourselves when we get these big initiatives is, ‘What’s the definition of success?’ ” he says.
Scientific discoveries alone don’t do it for him. Doctors need to build effective treatments — and patients need to follow along. This is the promise of precision medicine in the long run, but Joyner has published articles (like this one) skeptical of the drive to collect vast piles of information, hoping to make sense of it later.
“Is this just going to be a biological Tower of Babel,” he asks, or will this information also lead to discoveries that translate beyond the laboratory and actually change public health for the better?
He doesn’t doubt that DNA sequencing and other tools of precision medicine are useful in specific instances, such as for diagnosing rare diseases. There are a few uses in cancer treatment, but so far there has been just one randomized study of patients assigned to treatments based on their tumor’s DNA profile, and it showed no advantage over traditional medical judgment.
This isn’t a popular point of view elsewhere at the Mayo Clinic, which is sinking hundreds of millions of dollars a year into precision medicine. The shiny new labs across campus seem a world apart from Joyner’s physiology lab.
After my conversation with Joyner, Mayo public affairs officers eagerly led me to a series of scientists there who disagree with him. One stop was at the Medical Genome Facility.
This room is chock-full of machines that cost many hundreds of thousands of dollars apiece. They can analyze DNA from a person, a tumor or even a single human cell. They can read out an entire genome, or just a subset, which is called the exome.
“I really do believe that understanding our genome is fundamental,” says Julie Cunningham, one of the facility’s three co-directors. Like other scientists at Mayo, she has had swaths of her own exome sequenced.
“I learned that by and large I’m really lucky, but I learned that I have two [genetic] variants that affect how I respond to particular drugs that are out there.” She had actually known that before for one of those drugs, “but now I know exactly why I have it and it’s potentially serious. That has to be good. There’s nothing wrong with that.”
Bad drug reactions are a serious medical problem, so in principle, a genome profile should help reduce that risk. The reality, though, is that the vast majority of genomic tests for drug sensitivity give ambiguous answers.
One of the most carefully studied examples involves the blood thinner warfarin. Four separate studies show that people who got genomic testing for this susceptibility did no better — or only marginally better — than patients whose dosages were determined by traditional strategies.
This lack of strong data is a problem for many areas of precision medicine.
“Although we think conceptually this is going to be of high value, and it will ultimately improve health care with better quality and lower cost, we have to prove that,” says Keith Stewart, a hematologist who directs Mayo’s Center for Individualized Medicine. “If it doesn’t all pan out the way we expect that’s OK. But I think there will be plenty of unexpected areas that we haven’t conceptualized yet, so I think it’s a very worthwhile investment on the taxpayers’ point of view.”
Whether precision medicine is going to be a boom or a bust, “right now it’s just speculation on both sides,” he says.
“It is somewhat inevitable that every person in this country will have their genome sequenced.” he adds.
Gianrico Farrugia, a gastroenterologist who is chief executive officer of the Mayo Clinic in Florida, agrees.
“What we’re beginning to see is that technology is invading and replacing traditional testing,” says Farrugia. For example, it is already used to determine treatments for lung cancer and diagnose rare diseases.
And, in any event, he says medicine is already moving aggressively into this new territory and there’s no going back. “That’s not only wrong,” he says, “it’s impossible.”
With the momentum now built up behind this enterprise, precision medicine will be judged like much of modern medicine: in hindsight, after it becomes entrenched in clinical practice.
You can contact Richard Harris via email.
9(MDAzMzI1ODY3MDEyMzkzOTE3NjIxNDg3MQ001))